Advertisements
Advertisements
Question
Calculate the half-life of a first order reaction from the rate constant given below:
200 s−1
Solution
`t_(1/2) = 0.693/k`
= `0.693/200`
= 3.46 × 10 −3 s
APPEARS IN
RELATED QUESTIONS
The integrated rate equation for first order reaction is A → products
Derive the relation between half life and rate constant for a first order reaction
The rate constant for a first order reaction is 100 s–1. The time required for completion of 50% of reaction is _______.
(A) 0.0693 milliseconds
(B) 0.693 milliseconds
(C) 6.93 milliseconds
(D) 69.3 milliseconds
The experimental data for decomposition of N2O5
\[\ce{2N2O5 -> 4NO2 + O2}\] in gas phase at 318K are given below:
| t/s | 0 | 400 | 800 | 1200 | 1600 | 2000 | 2400 | 2800 | 3200 |
| 102 × [N2O5]/mol L−1 | 1.63 | 1.36 | 1.14 | 0.93 | 0.78 | 0.64 | 0.53 | 0.43 | 0.35 |
- Plot [N2O5] against t.
- Find the half-life period for the reaction.
- Draw a graph between log [N2O5] and t.
- What is the rate law?
- Calculate the rate constant.
- Calculate the half-life period from k and compare it with (ii).
During nuclear explosion, one of the products is 90Sr with half-life of 28.1 years. If 1μg of 90Sr was absorbed in the bones of a newly born baby instead of calcium, how much of it will remain after 10 years and 60 years if it is not lost metabolically.
Which among the following reactions is an example of a zero order reaction?
a) `H_(2(g)) + I_(2(g)) -> 2HI_(g)`
b) `2H_2O_(2(l)) -> 2H_2O_(l) + O_(2(g))`
c) `C_12H_22O_(11(aq)) + H_2O_(l) -> C_6H_12O_(6(aq)) + C_6H_12O_(6(aq))`
d) `2NH_(3g)` `N(2g) + 3H_(2(g))`
The half-life period of zero order reaction A → product is given by
(a) `([A]_0)/k`
(b) `0.693/k`
(c) `[A]_0/(2k)`
(d) `(2[A]_0)/k`
For the first order reaction, half-life is equal to ____________.
Calculate half-life period of life order reaction whose rate constant is 200 sec–1
Which radioactive isotope would have the longer half-life 15O or 19O? (Given rate constants for 15O and 19O are 5.63 × 10–3 s–1 and k = 2.38 × 10–2 s–1 respectively.)
A first-order reaction takes 69.3 min for 50% completion. What is the time needed for 80% of the reaction to get completed? (Given: log 5 = 0.6990, log 8 = 0.9030, log 2 = 0.3010)
Observe the graph shown in figure and answer the following questions:
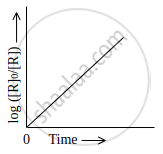
Write the relationship between k and t1/2 (half-life period)
The half-life of cobalt 60 is 5.26 years. The percentage activity remaining after 4 years is ______%.
The rate of a first order reaction is 0.04 mol litre-1 s-1 at 10 minutes and 0.03 mol litre-1 sec-1 at 20 minutes after initiation. The half-life of the reaction is ______ min.
A first order reaction takes 40 min for 30% decomposition. Calculate `"t"_(1/2)`.
