Advertisements
Advertisements
Question
Can a hydrogen atom emit characteristic X-rays?
Solution
The difference of energy levels in a hydrogen atom is small. Hence, it is not able to emit characteristic X-rays.
APPEARS IN
RELATED QUESTIONS
To which part of the electromagnetic spectrum does a wave of frequency 5 × 1011 Hz belong?
What physical quantity is the same for X-rays of wavelength 10−10 m, red light of wavelength 6800 Å and radiowaves of wavelength 500 m?
Given below are some famous numbers associated with electromagnetic radiations in different contexts in physics. State the part of the electromagnetic spectrum to which each belongs.
(a) 21 cm (wavelength emitted by atomic hydrogen in interstellar space).
(b) 1057 MHz (frequency of radiation arising from two close energy levels in hydrogen; known as Lamb shift).
(c) 2.7 K [temperature associated with the isotropic radiation filling all space-thought to be a relic of the ‘big-bang’ origin of the universe].
(d) 5890 Å - 5896 Å [double lines of sodium]
(e) 14.4 keV [energy of a particular transition in 57Fe nucleus associated with a famous high resolution spectroscopic method (Mössbauer spectroscopy)].
Give one use of gamma rays.
The energy of a photon of a characteristic X-ray from a Coolidge tube comes from
For harder X-rays,
(a) the wavelength is higher
(b) the intensity is higher
(c) the frequency is higher
(d) the photon energy is higher.
The wavelength of Kα X-ray of tungsten is 21.3 pm. It takes 11.3 keV to knock out an electron from the L shell of a tungsten atom. What should be the minimum accelerating voltage across an X-ray tube having tungsten target which allows production of Kα X-ray?
(Use Planck constant h = 6.63 × 10-34 Js= 4.14 × 10-15 eVs, speed of light c = 3 × 108 m/s.)
The stopping potential in a photoelectric experiment is linearly related to the inverse of the wavelength (1/λ) of the light falling on the cathode. The potential difference applied across an X-ray tube is linearly related to the inverse of the cutoff wavelength (1/λ) of the X-ray emitted. Show that the slopes of the lines in the two cases are equal and find its value.
(Use Planck constant h = 6.63 × 10-34 Js= 4.14 × 10-15 eVs, speed of light c = 3 × 108 m/s.)
Name the scientist who discovered
X-rays
Answer the following question.
Gamma rays and radio waves travel with the same velocity in free space. Distinguish between them in terms of their origin and the main application.
State three properties of infrared radiations similar to that of visible light.
Give one use of electromagnetic radiation in Ultraviolet radiation.
What are the ultraviolet rays?
Answer briefly.
Give two uses of ultraviolet rays.
Answer briefly.
Give two uses of radio waves.
Name the e.m. waves which are suitable for radar systems used in aircraft navigation. Write the range of frequency of these waves.
Below is an incomplete table showing the arrangement of electromagnetic spectrum in the increasing order of their wavelength. Complete the table:
| Gamma ray | X - ray | UV rays | Visible rays | Infrared | A | Radio waves |
- Identify the radiation A.
- Name the radiation used to detect fracture in bones.
- Name one property common to both A and Radio waves.
In an atom X, electrons absorb the energy from an external source. This energy “excites” the electrons from a lower-energy level to a higher-energy level around the nucleus of the atom. When electrons return to the ground state, they emit photons.
The figure below is the energy level diagram of atom X with three energy levels, E1 = 0.00eV, E2 = 1.78eV and E3 = 2.95eV. The ground state is considered 0 eV for reference. The transition of electrons takes place between levels E1 and E2.
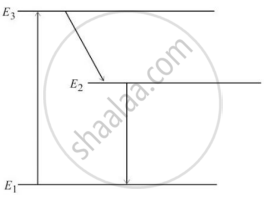
- What wavelength of radiation is needed to excite the atom to energy level E2 from E1?
- Suppose the external source has a power of 100 W. What would be the rate of photon emission?
