Advertisements
Advertisements
Question
Can Lα X-ray of one material have shorter wavelength than Kα X-ray of another?
Solution
An Lα X-ray is emitted when an electron jumps from the M to the L shell, and a Kα X-ray is emitted when an electron jumps from the L to the K shell. Less energy is involved when an electron jumps from the M to the L shell than when it jumps from the L to the K shell. Also, wavelength of a photon is inversely related to its energy. Therefore, an Lα X-ray has higher wavelength than a Kα X-ray for the same material.
APPEARS IN
RELATED QUESTIONS
(a) Give a list of at least five radiations, in order of their increasing frequencies, which make up the complete electromagnetic spectrum.
(b) Which of the radiation mentioned by you in part (a) has the highest penetrating power.
What is the range of the wavelength of the following electromagnetic waves?
(a) Gamma rays.
Name the region beyond the red end of the spectrum.
State the approximate range of wavelength associated with infrared rays.
Why is exposure to X-rays injurious to health but not exposure to visible light, when both are electromagnetic waves?
For a given material, the energy and wavelength of characteristic X-rays satisfy
(a) E(Kα) > E(Kβ) > E(Kγ)
(b) E(Mα) > E(Lα) > E(Kα)
(c) λ(Kα) > λ(Kβ) > λ(Kγ)
(d) λ(Mα) > λ(Lα) > λ(Kα).
Iron emits Kα X-ray of energy 6.4 keV. Calculate the times taken by an iron Kα photon to cross through a distance of 3 km.
(Use Planck constant h = 4.14 × 10-15 eVs, speed of light c = 3 × 108 m/s.)
The X-ray coming from a Coolidge tube has a cutoff wavelength of 80 pm. Find the kinetic energy of the electrons hitting the target.
(Use Planck constant h = 6.63 × 10-34 Js= 4.14 × 10-15 eVs, speed of light c = 3 × 108 m/s.)
Find the maximum potential difference which may be applied across an X-ray tube with tungsten target without emitting any characteristic K or L X-ray. The energy levels of the tungsten atom with an electron knocked out are as follows.
| Cell containing vacancy | K | L | M |
| Energy in keV | 69.5 | 11.3 | 2.3 |
Heat at the rate of 200 W is produced in an X-ray tube operating at 20 kV. Find the current in the circuit. Assume that only a small fraction of the kinetic energy of electrons is converted into X-rays.
Write the range of the wavelength of the following electromagnetic radiations:
(a) Infrared rays
(b) Ultraviolet rays
(c) γ -rays
Write one use of each of the above.
Two waves A and B have wavelength 0.01 Å and 9000 Å respectively.
Name the two waves. compare the speeds of these waves when they travel in vacuum.
Name the radiation which can be detected by thermopile.
How will you investigate the existence of the radiation beyond the red and violet extremes of the spectrum?
If the Earth did not have atmosphere, would its average surface temperature be higher or lower than what it is now? Explain.
Following QN ∴ 14, the radiation force on the roof will be
What is time period of the light for which the eye is most sensitive?
What happens to the intensity of light from a bulb if the distance from the bulb is doubled? As a laser beam travels across the length of a room, its intensity essentially remains constant. What geometrical characteristic of LASER beam is responsible for the constant intensity which is missing in the case of light from the bulb?
The half-value thickness of an absorber is defined as the thickness that will reduce exponentially the intensity of a beam of particles by a factor of 2. The half-value thickness in (µm) for lead assuming X-ray beam of wavelength 20 pm, µ = 50 cm-1 for X-rays in lead at wavelength λ = 20 pm, is ______ µm.
In an atom X, electrons absorb the energy from an external source. This energy “excites” the electrons from a lower-energy level to a higher-energy level around the nucleus of the atom. When electrons return to the ground state, they emit photons.
The figure below is the energy level diagram of atom X with three energy levels, E1 = 0.00eV, E2 = 1.78eV and E3 = 2.95eV. The ground state is considered 0 eV for reference. The transition of electrons takes place between levels E1 and E2.
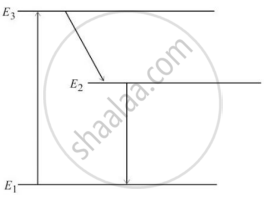
- What wavelength of radiation is needed to excite the atom to energy level E2 from E1?
- Suppose the external source has a power of 100 W. What would be the rate of photon emission?
