Advertisements
Advertisements
Question
Choose the correct answer:
The graph of PV vs P for gas is
Options
Parabolic
Hyperbolic
A straight line parallel to the X-axis
A straight line passing through the origin
Solution
Straight-line parallel to the X-axis.
APPEARS IN
RELATED QUESTIONS
Molar volume is the volume occupied by 1 mol of any (ideal) gas at standard temperature and pressure (STP: 1 atmospheric pressure, 0 °C). Show that it is 22.4 litres
The figure shows the plot of PV/T versus Pfor 1.00×10–3 kg of oxygen gas at two different temperatures.
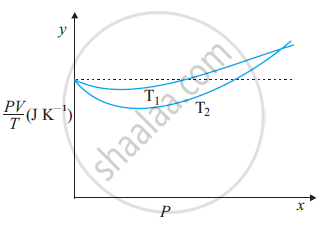
(a) What does the dotted plot signify?
(b) Which is true: T1 > T2 or T1 < T2?
(c) What is the value of PV/T where the curves meet on the y-axis?
(d) If we obtained similar plots for 1.00 ×10–3 kg of hydrogen, would we get the same value of PV/T at the point where the curves meet on the y-axis? If not, what mass of hydrogen yields the same value of PV/T (for low pressure high temperature region of the plot)? (Molecular mass of H2 = 2.02 u, of O2 = 32.0 u, R = 8.31 J mo1–1 K–1.)
Oxygen is filled in a closed metal jar of volume 1.0 × 10−3 m3 at a pressure of 1.5 × 105Pa and temperature 400 K. The jar has a small leak in it. The atmospheric pressure is 1.0 × 105 Pa and the atmospheric temperature is 300 K. Find the mass of the gas that leaks out by the time the pressure and the temperature inside the jar equalise with the surrounding.
Fill in the blanks:
If the temperature is reduced to half, ………….. would also reduce to half.
Name or state the following:
The absolute temperature value corresponding to 35°C.
The average energy per molecule is proportional to ______
Show that for diatomic gas the ratio of the two specific heats is 7:5.
The equation of state for 2g of oxygen at a pressure 'P' and temperature 'T', when occupying a volume 'V' will be ______.
A box of 1.00 m3 is filled with nitrogen at 1.50 atm at 300K. The box has a hole of an area 0.010 mm2. How much time is required for the pressure to reduce by 0.10 atm, if the pressure outside is 1 atm.
Two tanks of equal volume contain equal mass of oxygen and nitrogen at 127°C. Find the ratio of pressure in two tanks.
