Advertisements
Advertisements
Question
Classify the following substance:
Dilute hydrochloric acid
Options
Strong electrolyte
Weak electrolyte
Non-electrolyte
Solution
Strong electrolyte
RELATED QUESTIONS
Name a base which is a weak electrolyte.
Explain the following:
Hydrochloric acid is a good conductor of electricity.
Explain the term : Anode mud
What are the particles present in a compound which is a non- electrolyte?
Fill in the blank :
The _______ the concentration of an ion in a solution, the greater is the probability of its being discharged at its appropriate electrode.
Three different electrolytic cells, A, B and C are connected in separate circuits. Electrolytic cell A contains a sodium chloride solution. When the circuit is completed, a bulb in the circuit glows brightly. Electrolytic cell B contains an acetic acid solution and in this case, the bulb in the circuit glows dimly. The electrolytic cell C contains a sugar solution and the bulb does not glow. Give a reason for each of these observations.
Fill in the blank from the choices given below :
Electro covalent compounds have a _____ boiling point
Copper sulphate solution is electrolyzed using copper electrodes. Study the diagram given alongside and answer the questions that follow.
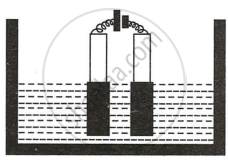
- Which electrode to your left or right is known as the oxidizing electrode and why?
- Write the equation representing the reaction that occurs.
- State two appropriate observations for the above electrolysis reactions.
Give appropriate scientific reasons for the following statement :
Zinc oxide can be reduced to zinc by using carbon monoxide, but aluminium oxide cannot be reduced by a reducing agent.
Copper sulphate solution is electrolysed using copper electrodes.
Write the equation for the reaction occurring at the Anode electrode.
