Advertisements
Advertisements
Question
CO2 and H2O both are triatomic molecule but their dipole moment values are different. Why?
Solution
- A linear form of carbon dioxide has zero dipole moment. In CO2 the dipole moment of two polar bonds are equal in magnitude but have opposite directions. Hence, the net dipole moment of the CO2 molecule is
µ = µ1 + µ2
µ = µ1 + (- µ1) = 0
In this case `mu = vec mu_1 + vec((- mu_1) = 0` - But in the case of water, net dipole moment is the vector sum µ1 + µ2 as follows:
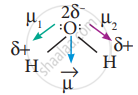
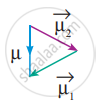
Dipole moment in water is found to be 1.85 D.
- CO2 and H2O both are triatomic molecules but their dipole moment values are zero and 1.85 D respectively.
APPEARS IN
RELATED QUESTIONS
Bond order of a species is 2.5 and the number of electons in its bonding molecular orbital is formd to be 8 The no. of electons in its antibonding molecular orbital is
Define bond order.
What is dipole moment?
Define bond energy.
Explain resonance with reference to a carbonate ion.
Explain the bond formation in ethylene.
Which one of the following has the highest bond order?
- N2
- N2+
- N2–
Describe Fajan’s rule.
The correct sequence of decrease in the bond angles of the following hydrides is.
Bond distance in HF is 9.17 × 10−11 m. Dipole moment of HF is 6.104 × 10−30 Cm. The percentage of ionic character in HF will be ______.
(electron charge = 1.60 × 10−19 C)
