Advertisements
Advertisements
Question
Complete flow chart given below.
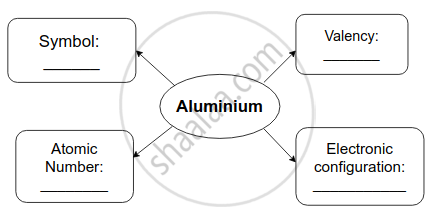
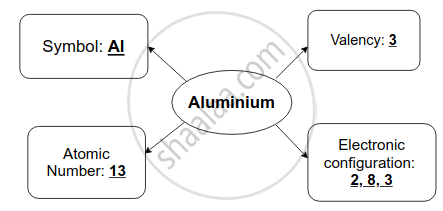
Solution
RELATED QUESTIONS
The process by which sulphide ore is concentrated.
How many carats is pure gold? Why is pure gold not suitable for making ornaments?
Silver metal does not combine easily with oxygen but silver jewellery tarnishes after some time. How?
Explain why, an aqueous solution of sodium chloride is not used for the electrolytic extraction of sodium metal.
Name the gas produced when calamine ore is calcined.
Name the electrode at which aluminium metal is produced.
Which metal is extracted from bauxite ore?
During galvanisation, iron metal is given a thin coating of one of the following metals. This metal is:
(a) chromium
(b) tin
(c) zinc
(d) copper
Give the principles of the froth floatation process.
Give the chemical formula of :
Sodium aluminate
Name the following:
The mixture of materials fed into a furnace to extract a metal.
Name the following:
The substance added to get rid of gangue in the extraction of metal.
Name the following:
The process of heating a substance very strongly in such a way that it does not combine with oxygen.
Name the following:
A compound which is added to lower the fusion temperature of the electrolytic bath in the extraction of aluminium.
Which one of the following is not true of metal :
Metals are malleable and ductile
Froth floatation process is generally used to concentrate ______ ores.
Observe the figure and answer the following.

- Write the name of the method.
- What is used as anode and cathode in this method?
- Write the molecular formula and use of cryolite.
- Write anode reaction.
- Write cathode reaction.
Which of the following metals exist in their native state in nature?
(i) Cu
(ii) Au
(iii) Zn
(iv) Ag
2 mL each of concentrated HCl, HNO3 and a mixture of concentrated HCl and concentrated HNO3 in the ratio of 3 : 1 were taken in test tubes labelled as A, B and C. A small piece of metal was put in each test tube. No change occurred in test tubes A and B but the metal got dissolved in test tube C respectively. The metal could be
A mineral from which the metal can be extracted economically and conveniently is known as ______.
