Advertisements
Advertisements
Question
Correct the following statement:
0°C is equal to zero Kelvin.
Solution
-273°C is equal to zero Kelvin.
APPEARS IN
RELATED QUESTIONS
Three vessels of equal capacity have gases at the same temperature and pressure. The first vessel contains neon (monatomic), the second contains chlorine (diatomic), and the third contains uranium hexafluoride (polyatomic).
Is the root mean square speed of molecules the same in the three cases? If not, in which case is vrms the largest?
Oxygen is filled in a closed metal jar of volume 1.0 × 10−3 m3 at a pressure of 1.5 × 105Pa and temperature 400 K. The jar has a small leak in it. The atmospheric pressure is 1.0 × 105 Pa and the atmospheric temperature is 300 K. Find the mass of the gas that leaks out by the time the pressure and the temperature inside the jar equalise with the surrounding.
What do you understand by gas?
Match the following:
|
|
Column A |
Column B |
|
(a) |
cm3 |
(i) Pressure |
|
(b) |
Kelvin |
(ii) Temperature |
|
(c) |
Torr |
(iii) Volume |
|
(d) |
Boyle's law |
(iv) `"V"/"T" = ("V"_1)/("T"_1)` |
|
(a) |
Charles's law |
(v) `"PV"/"T" = ("P"_1 "V"_1)/"T"_1` |
|
|
|
(vi) PV = P1V1 |
Name or state the following:
The standard pressure of a gas in cm. of mercury corresponding to one atmospheric pressure.
Name or state the following:
The absolute temperature value corresponding to 35°C.
The equation of state for 2g of oxygen at a pressure 'P' and temperature 'T', when occupying a volume 'V' will be ______.
Which of the following diagrams (Figure) depicts ideal gas behaviour?
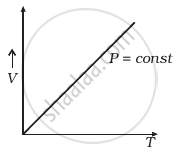 (a) |
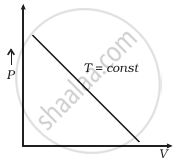 (b) |
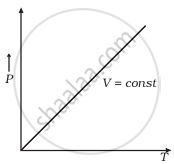 (c) |
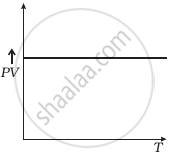 (d) |
Calculate the number of atoms in 39.4 g gold. Molar mass of gold is 197g mole–1.
Two tanks of equal volume contain equal mass of oxygen and nitrogen at 127°C. Find the ratio of pressure in two tanks.
