Advertisements
Advertisements
Question
Define: Atomic weight :
Solution
Atomic weight : It is the ratio that tells how many times an atom of an element is heavier than atom of Hydrogen
APPEARS IN
RELATED QUESTIONS
Write down the name of a compound represented by the following formula:
CaCl2
Write down the name of a compound represented by the following formula:
KNO3
Give the name of the element present in the following compound:
Hydrogen bromide
Calculate the number of molecules of sulphur (S8) present in 16 g of solid sulphur.
State the term.
The number of atoms present in a molecule of an element _________
Define: Atomic number
Define: Atomic number
Define: Mass number :
Write the formulae of the following compounds. Also name the elements present in them.
- Water
- Ammonia
- Methane
- Sulphur dioxide
- Ethanol
In what form does oxygen gas occur in nature ?
What is the difference between the molecule of an element and the molecule of a compound ? Give one
example of each.
One of the forms of a naturally occurring solid compound P is usually used for making the floors of houses. On adding a few drops of dilute hydrochloric acid to P, brisk effervescence are produced. When 50 g of reactant P was heated strongly, than 22 g of a gas Q and 28 g of a solid R were produced as products. Gas Q is the same which produced brisk effervescence on adding dilute HCl to P. Gas Q is said to cause global warming whereas solid R is used for white-washing.
- What is (i) solid P (ii) gas Q, and (iii) solid R.
- What is the total mass of Q and R obtained from 50 g of P ?
- How does the total mass of Q and R formed compare with the mass of P taken ?
- What conclusion do you get from the comparison of masses of products and reactant ?
- Which law of chemical combination is illustrated by the example given in this problem ?
Molecular compounds are usually formed by the combination between :
Calculate the mass of 3.011 × 1024 atoms of carbon.
Why are the symbols and formulae of substances important?
Explain the term – molecules. Give three examples of atoms of the same element forming a molecule. State the atomicity of the same.
State the atomicity of the following:
Neon
State the atomicity of the following:
Chlorine
State the atomicity of the following:
Argon
Which of the following is a short and scientific way of representing one molecule of an element or compound?
State true of false. If false, give the correct statement.
NaCl represents one molecule of sodium chloride.
Classify the following molecules as the molecules of element or compound
| 1. |  |
|
| 2. |  |
|
| 3. | 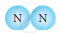 |
|
| 4. | 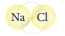 |
If a molecule is made of similar kinds of atoms, then it is called ______ atomic molecule.
Define: Atomicity.
