Advertisements
Advertisements
Question
Describe the role played in the extraction of aluminum:
Cryolite
Solution
In the extraction of aluminium, the given compounds play the following roles:
Cryolite: It lowers the fusion temperature from 2050°C to 950°C and enhances conductivity.
APPEARS IN
RELATED QUESTIONS
Name the process by which the refining of aluminium is done ?
Name the constituents of Bronze.
In order to obtain 1 tonne of aluminium, the following inputs are required: 4 tonnes of bauxite, 150 kg of sodium hydroxide and 600 kg of graphite. The aluminium compound in bauxite is aluminium oxide and the main impurity is iron (III) oxide. Aluminium is obtained by the electrolysis of aluminium oxide dissolved in cryolite.
When bauxite is treated with sodium hydroxide solution, what happens to:
- the aluminium oxide,
- the iron (III) oxide?
In the Hall's process for extraction of aluminium, Give the formula and purpose of fluorspar and cryolite
Name the following:
The middle region of the blast furnace.
The sketch below illustrates the refin ing of aluminium by Hoope's process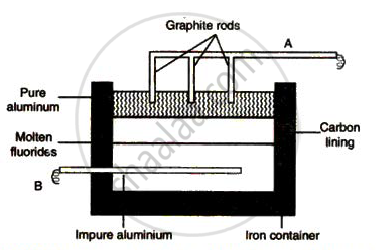
(a) Which of A and B is th e cathode and which one is the anode?
(b) What is the electroly te in the tank?
( c) What material is used for th e cathode?
The following question relate to the extraction of aluminium by electrolysis.
Name the other aluminium containing compound added to alumina and state the significance.
Name the following :
A compound added to lower the fusion temperature of electrolytic bath in the extracton of aluminium.
Name the alloy used for the following purpose.
Aircraft
Write the constituents of the electrolyte for the extraction of aluminium.
