Advertisements
Advertisements
Question
Describe the hybridisation in case of PCl5. Why are the axial bonds longer as compared to equatorial bonds?
Solution
The ground state and excited state outer electronic configurations of phosphorus (Z = 15) are:
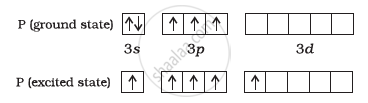
Phosphorus atom is sp3d hybridized in the excited state. These orbitals are filled by the electron pairs donated by five Cl atoms as:
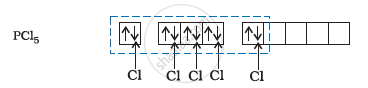
The five sp3d hybrid orbitals are directed towards the five corners of the trigonal bipyramidals. Hence, the geometry of PCl5 can be represented as:

There are five P–Cl sigma bonds in PCl5. Three P–Cl bonds lie in one plane and make an angle of 120° with each other. These bonds are called equatorial bonds.
The remaining two P–Cl bonds lie above and below the equatorial plane and make an angle of 90° with the plane. These bonds are called axial bonds.
As the axial bond pairs suffer more repulsion from the equatorial bond pairs, axial bonds are slightly longer than equatorial bonds.
APPEARS IN
RELATED QUESTIONS
Hybridisation of central atom in PCl5 involves the mixing of orbitals.
Shape and hybridisation of IF5 are ______.
The molecules having same hybridisation, shape and number of lone pairs of electons are ______.
Some of the following properties of two species, NO3– and H3O+ are described below. Which one of them is correct?
The types of hybridiration on the five carbon atom from right to left in the, 2,3 pentadiene.
The percentage of s-character of the hybrid orbitals in methane, ethane, ethene and ethyne are respectively.
Define Hybridisation.
In CH4, NH3, and H2O, the central atom undergoes sp3 hybridization – yet their bond angles are different. Why?
Explain Sp2 hybridization in BF3.
What type of hybridisation is possible in the following geometeries?
tetrahedral
What type of hybridisation is possible in the following geometeries?
square planer
Match the species in Column I with the geometry/shape in Column II.
| Column I | Column II |
| (i) \[\ce{H3O+}\] | (a) Linear |
| (ii) \[\ce{HC ≡ CH}\] | (b) Angular |
| (iii) \[\ce{ClO^{-}2}\] | (c) Tetrahedral |
| (iv) \[\ce{NH^{+}4}\] | (d) Trigonal bipyramidal |
| (e) Pyramidal |
In which of the following molecule does the central atom have two p-orbitals unused?
Which pair of species having identical shapes?
