Advertisements
Advertisements
Question
Differentiate between alkanes and alkenes. Name and draw the structure of one member of each.
Solution
(a) Alkanes: These are the simplest hydrocarbons having general formula CnH2n+2, where n is the number of atoms. These compounds contain all single covalent bonds between the carbon atoms. Suffix-ane is used while naming alkanes.
For e.g. C2H6
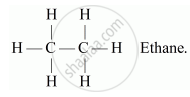
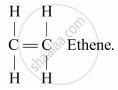
Alkenes: These are the simplest unsaturated hydrocarbons having general formula CnH2n, where n is the number of atoms. These compounds contain at least one double bond between the carbon atoms. Suffix, -ene is used while naming alkenes.
For e.g. C2H4
APPEARS IN
RELATED QUESTIONS
Draw the electron dot structures for ethanoic acid.
Write the electron-dot structures for ethene.
Fill in the following blank with suitable word:
The form of carbon which is used as a lubricant at high temperature is .........
Out of the following pairs of compounds, the unsaturated compounds are:
(a) C2H6 and C4H6
(b) C6H12 and C5H12
(c) C4H6 and C6H12
(d) C2H6 and C4H10
Which of the following hydrocarbons can decolourise bromine water and which cannot? Why?
C6H12, C6H14, C6H10
Fill in the blanks and rewrite the completed statements:
The organic compounds having double or triple bond in them are termed as _________________ _________________.
In which of the following compounds, — OH is the functional group?
The correct electron dot structure of a water molecule is
Carbon, Group (14) element in the Periodic Table, is known to form compounds with many elements. Write an example of a compound formed with
- chlorine (Group 17 of Periodic Table)
- oxygen (Group 16 of Periodic Table)
Identify and name the functional groups present in the following compounds.
- \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H - C - C - C - OH}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}\\
\end{array}\] - \[\begin{array}{cc}
\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{....}\\
\phantom{...}|\phantom{....}|\phantom{....}||\phantom{....}\\
\ce{H - C - C - C - OH}\\
\phantom{...}|\phantom{....}|\phantom{.........}\\
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{.......}\\
\end{array}\] - \[\begin{array}{cc}
\phantom{......}\ce{H}\phantom{....}\ce{H}\phantom{....}\ce{O}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{.......}\\
\phantom{....}|\phantom{....}|\phantom{....}||\phantom{....}|\phantom{....}|\phantom{....}\\
\ce{H} - \ce{C} - \ce{C} - \ce{C} - \ce{C} - \ce{C} - \ce{H}\\
\phantom{....}|\phantom{.....}|\phantom{........}|\phantom{....}|\phantom{....}\\
\phantom{....}\ce{H}\phantom{....}\ce{H}\phantom{........}\ce{H}\phantom{..}\ce{H}\phantom{.....}\\
\end{array}\] - \[\begin{array}{cc}
\phantom{......}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{......}\\
\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}\\
\ce{H - C - C - C = C - H}\\
\phantom{....}|\phantom{....}|\phantom{.............}\\
\phantom{....}\ce{H}\phantom{...}\ce{H}\phantom{.............}
\end{array}\]
