Advertisements
Advertisements
प्रश्न
Differentiate between alkanes and alkenes. Name and draw the structure of one member of each.
उत्तर
(a) Alkanes: These are the simplest hydrocarbons having general formula CnH2n+2, where n is the number of atoms. These compounds contain all single covalent bonds between the carbon atoms. Suffix-ane is used while naming alkanes.
For e.g. C2H6
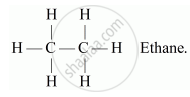
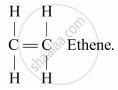
Alkenes: These are the simplest unsaturated hydrocarbons having general formula CnH2n, where n is the number of atoms. These compounds contain at least one double bond between the carbon atoms. Suffix, -ene is used while naming alkenes.
For e.g. C2H4
APPEARS IN
संबंधित प्रश्न
Define Unsaturated hydrocarbon.
Write the number of covalent bonds in the molecule of ethane.
What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Draw the electron dot structures for H2S.
Why are certain compounds called hydrocarbons? Write the general formula for homologous series of alkanes, alkenes and alkynes and also draw the structure of the first member of each series. Write the name of the reaction that converts alkenes into alkanes and also write a chemical equation to show the necessary conditions for the reaction to occur.
Out of the following pairs of compounds, the unsaturated compounds are:
(a) C2H6 and C4H6
(b) C6H12 and C5H12
(c) C4H6 and C6H12
(d) C2H6 and C4H10
Name the reaction which is usually used in the conversion of vegetables oils to fats. Explain the reaction involved in detail. Write a chemical equation to illustrate your answer.
Which of the following hydrocarbons can decolourise bromine water and which cannot? Why?
C6H12, C6H14, C6H10
Fill in the blanks and rewrite the completed statements:
The organic compounds having double or triple bond in them are termed as _________________ _________________.
