Advertisements
Advertisements
Question
Differentiate between:
Osmosis and Diffusion
Solution
| Osmosis | Diffusion |
| Osmosis is a special type of diffusion which involves the movement of solvent molecules from a region of their higher concentration to a region of their lower concentration through a semi-permeable membrane. | Diffusion is the movement of solute molecules or ions from a region of their higher concentration to a region of their lower concentration without the influence of a semi-permeable membrane. |
APPEARS IN
RELATED QUESTIONS
Name the following:
The condition of a cell placed in a hypotonic solution.
Complete the following statements:
Hypotonic solution is one in which the solution kept outside the cell has lower solute concentration than ……………… the cell.
Two potato cubes each 1 cm3 in size, were placed separately in two containers (A&B), the container (A) having water and the other (B) containing concentrated sugar solution. After 24 hours when the cubes were examined, those placed in water were found to be firm and had increased slightly in size and those placed in concentrated sugar solution were found to be soft and had somewhat decreased in size. Use the above information to answer the questions that follow:
Account for the firmness and increase in the size of the potato cubes placed in water.
Distinguish between the following:
Turgor pressure and osmotic pressure
Two potato cubes each 1 cm3 in size, were placed separately in two containers (A and B), the container A having water and the other (B) containing concentrated sugar solution. After 24 hours when the cubes were examined, those placed in water were found to be firm and had increased slightly in size and those placed in concentrated sugar solution were found to be soft and somewhat decreased in size. Use the above information to answer the questions that follow:
Account for the softness and decrease in size of the potato cubes which were placed in sugar solution.
The apparatus arranged here signifies an important process.
(i) Name the process.
(ii) Where does this process occur in plants?
(iii) What solution is placed inside the dialysis tubing?
(iv) What happens to the level of the solution in the capillary tube?
(v) Define the process mentioned in Q. (i) above.
In an experiment, two sets of apparatus were set up as shown below:
In A there is a concentrated sugar solution inside the thistle funnel and red ink in the water outside the funnel. In B there is a concentrated glucose solution with red ink inside the thistle funnel and water outside the thistle funnel.
In both A and B the level of liquid inside the funnels rises up the tubes. In A the sugar solution turns red and in B, the water turns red.
Study the given observations and answer these questions:

- Name the process by which red ink moves in A and B.
- Which type of pressure forces the water molecules to move towards thistle funnels and cause a rise in the water level?
- Where does this process occur in plants and animals?
- What material could be used as a semi-permeable membrane?
The beaker is divided into two chambers A and B. The big circle represents solute and the small circles solvent.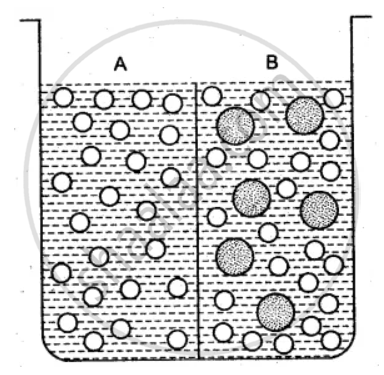
(i) What can you say about the size of the holes in the membrane, if it is to behave semi- permeably between these two?
(ii) Will the solvent molecules pass through the membrane from left to right, from right to left, in either direction or in both directions?
(iii) In which direction will there be a net movement of solvent molecules?
During a practical exam, a plant cell in a particular solution was placed under a compound microscope. Students were told to observe the cell and name the tonicity of the solution and mention the process that occurred in the cell.

