Advertisements
Advertisements
Question
Solution
Difference between mercury and alcohol as a thermometric substance
| Mercury | Alcohol |
| 1. It is non-volatile | It is volatile |
| 2. It has uniform expansion | It has non-uniform expansion |
| 3. It is opaque making it easier to read the thermometer | It is transparent thus making it difficult to read the thermometer |
| 4. It has a low coefficient of expansion | It has a high coefficient of expansion |
| 5. It has a high boiling point so it can be used to measure high temperatures | It has a low boiling point so it cannot be used to measure high temperatures |
APPEARS IN
RELATED QUESTIONS
Give reason for Sea water does not freeze at 0°C.
Explain the following:
How can you relate the formation of water droplets on the outer surface of a bottle taken out of refrigerator with formation of dew?
A 50 kg man is running at a speed of 18 km h−1. If all the kinetic energy of the man can be used to increase the temperature of water from 20°C to 30°C, how much water can be heated with this energy?
At what temperature is the density of water is maximum? State its value.
Water is cooled from 4 °C to 0 °C. It will :
Explain why do vegetables and fruits get damaged during severe frost?
While studying anomalous behaviour of water in Hope’s apparatus, the upper temperature of the thermometer : 0 °C : : lower temperature of the thermometer : _______
Write scientific reason.
Fish can survive even in frozen ponds in cold regions.
Observe the following diagram and write the answers to the questions given below.
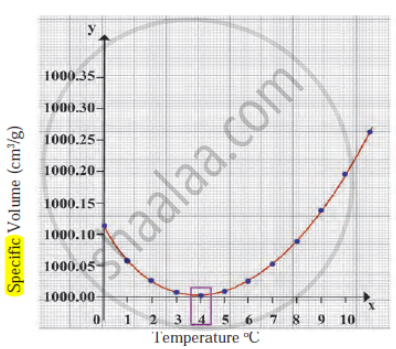
- Which process does the graph represent?
- What is the range of temperature responsible for the behaviour?
