Advertisements
Advertisements
Question
Water is cooled from 4 °C to 0 °C. It will :
Options
contract
expand
first contract, then expand
first expand, then contract
Solution
Water shows anomalous behavior between 0 °C and 4 °C. Hence, when it is cooled it expands.
APPEARS IN
RELATED QUESTIONS
Explain the following:
What is the role of the anomalous behaviour of water in preserving aquatic life in regions of cold climate?
Explain the following:
How can you relate the formation of water droplets on the outer surface of a bottle taken out of refrigerator with formation of dew?
Calculate the time required to heat 20 kg of water from 10°C to 35°C using an immersion heater rated 1000 W. Assume that 80% of the power input is used to heat the water. Specific heat capacity of water = 42000 J kg−1 K−1.
On a winter day the temperature of the tap water is 20°C whereas the room temperature is 5°C. Water is stored in a tank of capacity 0.5 m3 for household use. If it were possible to use the heat liberated by the water to lift a 10 kg mass vertically, how high can it be lifted as the water comes to the room temperature? Take g = 10 m s−2.
A 50 kg man is running at a speed of 18 km h−1. If all the kinetic energy of the man can be used to increase the temperature of water from 20°C to 30°C, how much water can be heated with this energy?
A deep pond of water has its top layer frozen. What will be the likely temperature of water layer at the bottom of the pond?
Why does a thick glass tumbler crack when very hot water is poured in it?
Study the following diagrams and write down your observations.
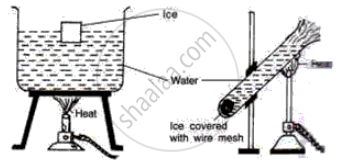
Write a short note.
Anomalous behaviour of water
