Advertisements
Advertisements
Question
Draw the TP diagram (P-x axis, T-y axis), VT(T-x axis, V-y axis) diagram for
- Isochoric process
- Isothermal process
- Isobaric process
Solution
a. Isobaric process: PV0 = nRT

P(T) = `("nRT")/"V"_0` and T(V) = Multivalued
b. Isothermal process: PV = nRT0
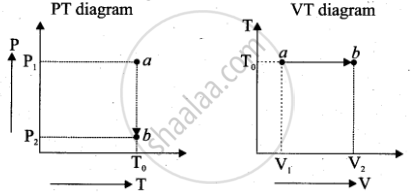
P(T) = Multivalued and T(V) = T0
c. Isobaric process: P0V = nRT
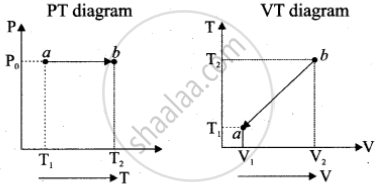
P(T) = P0 and T(V) = `("P"_0"V")/"nR"`
APPEARS IN
RELATED QUESTIONS
Heating a gas in a constant volume container is an example of which process?
Explain graphically (i) positive work with varying pressure, (ii) negative work with varying pressure, and (iii) positive work at constant pressure.
When you exercise in the morning, by considering your body as a thermodynamic system, which of the following is true?
Give an expression for work done in an isothermal process.
Draw the PV diagram for the isothermal process.
Can the given heat energy be completely converted to work in a cyclic process? If not, when can the heat can completely converted to work?
A monoatomic gas of pressure p having volume V expands isothermally to a volume 2V and then adiabatically to a volume 16V. The final pressure of the gas is ____________.
`("ratio of specific heats" = 5/3)`
Two identical samples of a gas are allowed to expand (i) isothermally (ii) adiabatically. Work done is ____________.
An ideal gas is compressed to half its initial volume by means of several processes. Which of the process results in the maximum work done on the gas?
Ideal gas for which 'ϒ' = 1.5 is suddenly compressed to `1/4`th of its initial volume. The ratio of 4 the final pressure to the initial pressure is ______.
`(ϒ = "C"_"p"/"C"_"v")`
