Advertisements
Advertisements
प्रश्न
Draw the TP diagram (P-x axis, T-y axis), VT(T-x axis, V-y axis) diagram for
- Isochoric process
- Isothermal process
- Isobaric process
उत्तर
a. Isobaric process: PV0 = nRT

P(T) = `("nRT")/"V"_0` and T(V) = Multivalued
b. Isothermal process: PV = nRT0
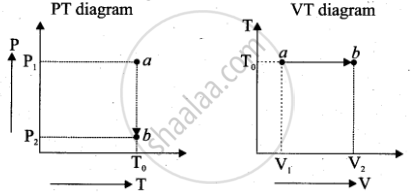
P(T) = Multivalued and T(V) = T0
c. Isobaric process: P0V = nRT
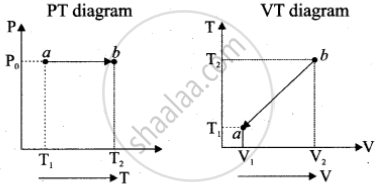
P(T) = P0 and T(V) = `("P"_0"V")/"nR"`
APPEARS IN
संबंधित प्रश्न
Heating a gas in a constant volume container is an example of which process?
Explain the cyclic process.
3 mole of a gas at temperature 400 K expands isothermally from an initial volume of 4 litres to a final volume of 8 litres. Find the work done by the gas. (R = 8.31 J mol-1 K-1)
Give an equation state for an isochoric process.
Explain in detail an adiabatic process.
Explain the isobaric process and derive the work done in this process.
For a given ideal gas 6 × 105 J heat energy is supplied and the volume of gas is increased from 4 m3 to 6 m3 at atmospheric pressure. Calculate
- the work done by the gas
- change in internal energy of the gas
- graph this process in PV and TV diagram
An ideal gas is made to go from a state A to stale B in the given two different ways (see figure) (i) an isobaric and then an isochoric process and (ii) an isochoric and then an isobaric process. The work done by gas in the two processes are W1 and W2 respectively. Then,

In which of the following processes, beat is neither absorbed nor released by a system?
For an isothermal expansion of a perfect gas, the value of `(Delta "P")/"P"` is equal to ____________.
