Advertisements
Advertisements
Question
Write short notes on the following Coupling reaction
Solution 1
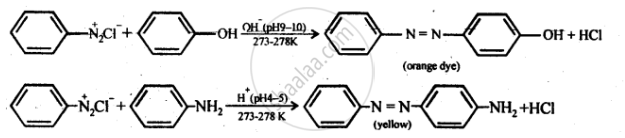
Coupling reaction - The reaction of joining two aromatic rings through the −N=N−bond is known as coupling reaction. Arenediazonium salts such as benzene diazonium salts react with phenol or aromatic amines to form coloured azo compounds.

It can be observed that, the para-positions of phenol and aniline are coupled with the diazonium salt. This reaction proceeds through electrophilic substitution.
Solution 2
Coupling reaction: In this reaction, arene diazonium salt reacts with aromatic amino compound (in acidic medium) or a phenol (in alkaline medium) to form brightly coloured azo compounds. The reaction generally takes place at para position to the hydroxy or amino group. If para position is blocked, it occurs at ortho position and if both ortho and para positions are occupied, than no coupling takes place.
APPEARS IN
RELATED QUESTIONS
Illustrate the following reactions giving suitable example in each case
Coupling reaction
Write equations of the following reactions:
Coupling reaction
Which of the following compound will not undergo azo coupling reaction with benzene diazonium chloride?
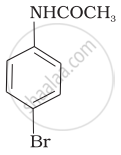
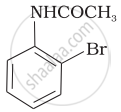
The product of the following reaction is:
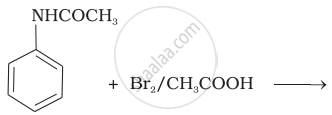
(i)
(ii)
(iii)
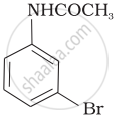
(iv)
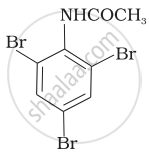
Which of the following reactions belong to electrophilic aromatic substitution?
(i) Bromination of acetanilide
(ii) Coupling reaction of aryldiazonium salts
(iii) Diazotisation of aniline
(iv) Acylation of aniline
Under what reaction conditions (acidic/basic), the coupling reaction of aryldiazonium chloride with aniline is carried out?
Benzenediazonium chloride reacts with phenol to give p-hydroxy azobenzene, an orange dye. This reaction is known as ______.
When benzene diazonium chloride reacts with phenol, it forms a dye. This reaction is called ______.
