Advertisements
Advertisements
Question
Elements \[\ce{X, Y}\] and \[\ce{Z}\] have 4, 5 and 7 valence electrons respectively. Which of these compounds will have the highest dipole moment?
Solution
Elements \[\ce{X, Y}\] and \[\ce{Z}\] having 4, 5 and 7 valence electrons respectively belongs to the second period and group 14th, 15th and 17th. The electronegativity of the elements increases on moving across the period and the dipole moment is calculated by the difference in the electronegativity of the atoms in a molecular structure. Higher the difference in the electronegativity will be the dipole moment. So, the dipole moment of \[\ce{H - Z}\] will be the highest.
APPEARS IN
RELATED QUESTIONS
Discuss the shape of the following molecules using the VSEPR model:
BeCl2, BCl3, SiCl4, AsF5, H2S, PH3
Although geometries of NH3 and H2O molecules are distorted tetrahedral, bond angle in water is less than that of ammonia. Discuss.
According to VSEPR theory, the repulsion between different parts of electrons obey the order.
Explain VSEPR theory. Applying this theory to predict the shapes of IF7 and SF6.
Identify the molecule with linear geometry?
Consider the species CH4, `"NH"_4^+` and `"BH"_4^-`. Choose the correct option with respect to these species.
Number of lone pair(s) of electrons on central atom and the shape of BrF3 molecule respectively are ______.
The number of lone pairs of electrons on the central I atom in `"I"_3^-` is ______.
In the following structure, the percentage of the 's' character in the lone pair occupy by the oxygen atom is ______.
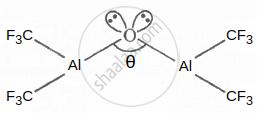
Given: Cos θ = −0.99
What is the geometry of a water molecule?
