Advertisements
Advertisements
Question
Discuss the shape of the following molecules using the VSEPR model:
BeCl2, BCl3, SiCl4, AsF5, H2S, PH3
Solution
BeCl2:
![]()
The central atom has no lone pair and there are two bond pairs. i.e., BeCl2 is of the type AB2. Hence, it has a linear shape.
BCl3:
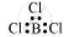
The central atom has no lone pair and there are three bond pairs. Hence, it is of the type AB3. Hence, it is trigonal planar.

SiCl4:
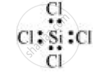
The central atom has no lone pair and there are four bond pairs. Hence, the shape of SiCl4 is tetrahedral being the AB4 type molecule.
AsF5:
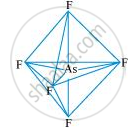
The central atom has no lone pair and there are five bond pairs. Hence, AsF5 is of the type AB5. Therefore, the shape is trigonal bipyramidal.
H2S:
![]()
The central atom has one lone pair and there are two bond pairs. Hence, H2S is of the type AB2E. The shape is Bent.
PH3:
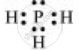
The central atom has one lone pair and there are three bond pairs. Hence, PH3 is of the AB3E type. Therefore, the shape is trigonal pyramidal.
APPEARS IN
RELATED QUESTIONS
Although geometries of NH3 and H2O molecules are distorted tetrahedral, bond angle in water is less than that of ammonia. Discuss.
According to VSEPR theory, the repulsion between different parts of electrons obey the order.
Shape of ClF3 is ______.
Identify the molecule with linear geometry?
Explain the non-linear shape of \[\ce{H2S}\] and non-planar shape of \[\ce{PCl3}\] using valence shell electron pair repulsion theory.
Elements \[\ce{X, Y}\] and \[\ce{Z}\] have 4, 5 and 7 valence electrons respectively. Write the molecular formula of the compounds formed by these elements individually with hydrogen.
Elements \[\ce{X, Y}\] and \[\ce{Z}\] have 4, 5 and 7 valence electrons respectively. Which of these compounds will have the highest dipole moment?
Which of the possible molecule/species is having maximum values for dipole moment. (where "A" is the central atom)?
Number of lone pair(s) of electrons on central atom and the shape of BrF3 molecule respectively are ______.
The number of lone pairs of electrons on the central I atom in `"I"_3^-` is ______.
Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R:
Assertion A: The H-O-H bond angle in water molecule is 104.5°.
Reason R: The lone pair-lone pair repulsion of electrons is higher than the bond pair-bond pair repulsion.
In the light of the above statements, choose the correct answer from the options given below:
In the following structure, the percentage of the 's' character in the lone pair occupy by the oxygen atom is ______.
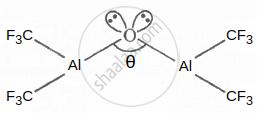
Given: Cos θ = −0.99
What is the geometry of a water molecule?
What is the number of lone pair of electrons in IF7?
Identify compound having square pyramidal-shape:from following.
