Advertisements
Advertisements
Question
Explain giving one example, how highly reactive metals (which are high up in the reactivity series) are extracted.
Solution
Sodium, potassium, calcium, magnesium and aluminium are highly reactive metals and are placed at the top of the reactivity series. These metals are extracted by electrolytic reduction of their molten chlorides or oxides because these metals are not reduced by other reducing agents like coke, carbon monoxide etc.
Let us take an example of extraction of sodium metal. Sodium metal is extracted by electrolytic reduction of molten sodium chloride. On passing electricity through molten sodium chloride, decomposition reaction occurs and formation of sodium metal and chlorine gas takes place.
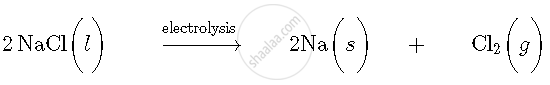
Molten sodium chloride consists of sodium and chloride ions. The reactions that occurs during electrolysis are:
1. Cathode produces electrons to reduce sodium ions to sodium atoms (or sodium metal) by acting as a reducing agent. Sodium ions are cations; therefore, they get attracted to negatively charged cathode and get deposited there.
Cathode: 2Na+ + 2e− → 2Na
Sodium ions Electrons Sodium atoms
(from molten NaCl) from cathode (or sodium metal)
2. Chloride ions, being anions, get attracted to positively charged anode. These chloride ions are oxidised to chlorine gas. Chlorine gas is produced at anode.
Anode: 2Cl− − 2e− → Cl2
Chloride ions electrons Chlorine gas
(from molten NaCl) to anode
APPEARS IN
RELATED QUESTIONS
M is a metal above hydrogen in the activity series and its oxide has the formula M2O. This oxide when dissolved in water forms the corresponding hydroxide which is a good conductor of electricity. In the above context, answer the following:
1) What kind of combination exists between M and O?
2)How many electrons are there in the outermost shell of M?
3) Name the group to which M belongs.
4) State the reaction taking place at the cathode.
5) Name the product at the anode.
Name one metal which is extracted by reduction with carbon.
Some metallic oxides can be reduced by hydrogen, carbon and carbon monoxide and some cannot. Explain.
Write equations for each of the above metal which reacts with water.
Name : The metal which is liquid at room temperature.
Which one of the following is not true of metal :
Metals form non-polar covalent compounds
What is the difference between calcination and roasting?
Write the molecular formulae of the following compound.
Cryolite
Explain the following reaction with the balanced equation.
Sodium aluminate reacts with water
The poorest conductor of heat among metals is ____________.
