Advertisements
Advertisements
Question
Describe with one example, how moderately reactive metals (which are in the middle of reactivity series) are extracted.
Solution
The oxides of moderately reactive metals like zinc, iron, lead copper, placed in the middle of the reactivity series, are reduced by carbon because carbon is more reactive.
Let us take an example of extraction of zinc from its carbonate ore, i.e., calamine.
The steps involved in the extraction of concentrated calamine ore are:
1. Carbonate of zinc is converted to its oxide by calcination process. In calcination process, zinc carbonate ore is heated strongly in the absence of air to produce zinc oxide and carbon dioxide gas

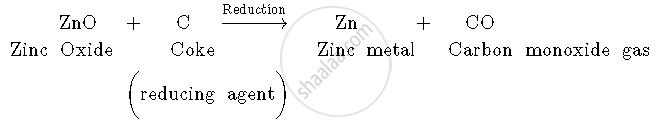
2. Zinc oxide is then reduced by coke (carbon). When zinc oxide is heated with coke, carbon acts as a reducing agent and reduces zinc oxide to zinc metal.
APPEARS IN
RELATED QUESTIONS
Define the Ore.
Why are the metals like Na, K, Ca and Mg never found in their free state in nature?
Explain giving one example, how highly reactive metals (which are high up in the reactivity series) are extracted.
What iron compound is present in haematite ore? Also write its chemical formula.
A sulphide ore is converted into metal oxide by the process of:
(a) carbonation
(b) roasting
(c) calcination
(d) anodising
Zinc blende ore can be converted into zinc oxide by the process of:
(a) roasting
(b) hydrogenation
(c) chlorination
(d) calcination
When an object made of metal A is kept in air for a considerable time, it loses its shine and becomes almost black due to the formation of a layer of substance B. When an object made of another metal C is kept in damp air for a considerable time, it gets covered with a green layer of substance D. Metal A is the best conductor of electricity whereas metal C is the next best conductor of electricity.
(a) What is metal A?
(b) What is metal C?
(c) Name the substance B.
(d) Name the substance D.
Give the principles of the hydrolytic method.
Name the methods by which concentrated ore is converted to metallic oxide.
Find the odd one out and give its explanation.
