Advertisements
Advertisements
Question
Explain the Structure of Diborane.
Solution
B2H6 is an electron-deficient compound. B2H6 has only 12 electrons – 6 e– from 6 H atoms and 3 e– each from 2 B atoms. Thus, after combining with 3 H atoms, none of the boron atoms has any electrons left. X-ray diffraction studies have shown the structure of diborane as:

2 boron and 4 terminal hydrogen atoms (Ht) lie in one plane, while the other two bridging hydrogen atoms (Hb) lie in a plane perpendicular to the plane of boron atoms. Again, of the two bridging hydrogen atoms, one H atom lies above the plane and the other lies below the plane. The terminal bonds are regular two-centre two-electron (2c – 2e–) bonds, while the two bridging (B–H–B) bonds are three-centre two-electron (3c – 2e–) bonds.
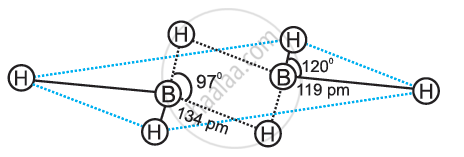
APPEARS IN
RELATED QUESTIONS
Explain the Structure of Boric Acid.
Explain the following reaction.
Hydrated alumina is treated with aqueous NaOH solution.
A certain salt X, gives the following results.
- Its aqueous solution is alkaline to litmus.
- It swells up to a glassy material Y on strong heating.
- When conc. H2SO4 is added to a hot solution of X, white crystal of an acid Z separates out.
Write equations for all the above reactions and identify X, Y and Z.
Write a balanced equation for \[\ce{H3BO3 ->[\Delta]}\]?
Boric acid is polymeric due to ______.
An aqueous solution of borax is _______.
Boric acid is an acid because its molecule ______.
Draw the structure of boric acid showing hydrogen bonding. Which species is present in water? What is the hybridisation of boron in this species?
When aqueous solution of borax is acidified with hydrochloric acid, a white crystalline solid is formed which is soapy to touch. Is this solid acidic or basic in nature? Explain.
What are boranes? Give chemical equation for the preparation of diborane.
Boric acid heated to red hot gives ______.
Which of the following compounds are formed when boron trichloride is treated with water?
Boron reacts with nitric acid to form ______.
Which one of the following methods is used to prepare borax crystals?
Borazine, also known as inorganic benzene, can be prepared by the reaction of 3-equivalents of “X” with 6-equivalents of “Y”. “X” and “Y”, respectively are ______.
The reaction of H3N3B3Cl3(A) with LiBH4 in tetrahydrofuran gives inorganic benzene (B). Further, the reaction of (A) with (C) leads to H3N3B3(Me)3. Compounds (B) and (C) respectively, are ______.
