Advertisements
Advertisements
Question
Draw the structure of boric acid showing hydrogen bonding. Which species is present in water? What is the hybridisation of boron in this species?
Solution
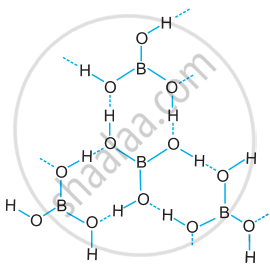
Structure of boric acid; the dotted lines represent hydrogen bonds
[B(OH)4]– units are present in water. Boron has sp3 hybridisation in [B(OH)4]– unit.
APPEARS IN
RELATED QUESTIONS
Explain the following reaction.
Hydrated alumina is treated with aqueous NaOH solution.
Write a balanced equation for B2H6 + H2O → ?
Write a balanced equation for \[\ce{H3BO3 ->[\Delta]}\]?
Boric acid is polymeric due to ______.
An aqueous solution of borax is _______.
Explain the Structure of Diborane.
Draw the structures of BCl3.NH3 and AlCl3 (dimer).
Boric acid heated to red hot gives ______.
On the addition of mineral acid to an aqueous solution of borax, the compound formed is ______.
Borazine, also known as inorganic benzene, can be prepared by the reaction of 3-equivalents of “X” with 6-equivalents of “Y”. “X” and “Y”, respectively are ______.
