Advertisements
Advertisements
Question
Explain why is OH group in phenols more strongly held as compared to OH group in alcohols.
Solution
In phenol, oxygen atom is attached to sp2-hybridised carbon atom while in alcohol, it is attached to sp3-hybridised carbon atom. The bond formed between oxygen and sp2-hybridised carbon is more strongly held then that formed between oxygen and sp3-hybridised carbon.
APPEARS IN
RELATED QUESTIONS
Write the mechanism of the following reaction :
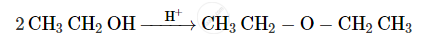
Give the equation of the following reaction:
Oxidation of propan-1-ol with alkaline KMnO4 solution.
Lucas reagent is ____________.
Which one of the following on oxidation gives a ketone?
Which of the following alcohols will not undergo oxidation?
Write the mechanism of acid dehydration of ethanol to yield ethene.
Write the mechanism of acid dehydration of ethanol to yield ethene.
Write the mechanism of acid-catalysed dehydration of ethanol to yield ethene.
Write the mechanism of acid-catalysed dehydration of ethanol to yield ethene.
Write the mechanism of acid-catalysed dehydration of ethanol to yield ethene
