Advertisements
Advertisements
Question
Find the binding energy per nucleon of 235U based on the information given below.
| Mass(u) | |
| mass of neutral `""_92^235"U"` | 235.0439 |
| mass of a proton | 1.0073 |
| mass of a neutron | 1.0087 |
Solution
mass of protons (mp) = 92 × 1.0073u = 92.6716u
mass of neutrons (mn) = 143 × 1.0087u = 144.2441u
Total mass (mp + mn) = 236.9157u
mass defect = Δm = 236.9157u – 235.0439u
=1·8718u
Binding energy = Δm × 931.5
MeV = 1.8718 × 931.5
MeV = 1743.6 MeV
Binding energy per nucleon = `(1743.6 "MeV")/ 235`
= 7.42 MeV
APPEARS IN
RELATED QUESTIONS
Write symbolically the nuclear β+ decay process of `""_6^11C` Is the decayed product X an isotope or isobar of (`""_6^11C`)? Given the mass values m (`""_6^11C`) = 11.011434 u and m (X) = 11.009305 u. Estimate the Q-value in this process.
What is the significance of binding energy per nucleon of a nucleus of a radioactive element?
Define half-life of a radioactive substance
Define the terms (i) half-life (T1/2) and (ii) average life (τ). Find out their relationships with the decay constant (λ).
In a nuclear reactor, what is the function of:
(i) The moderator
(ii) The control rods
(iii) The coolant
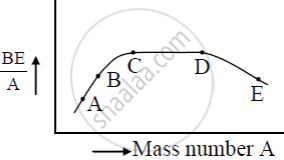
The figure shows the plot of binding energy (BE) per nucleon as a function of mass number A. The letters A, B, C, D, and E represent the positions of typical nuclei on the curve. Point out, giving reasons, the two processes (in terms of A, B, C, D, and E ), one of which can occur due to nuclear fission and the other due to nuclear fusion.
Calculate the binding energy of an alpha particle given its mass to be 4.00151 u.
The difference in mass of a nucleus and its constituents is called ______.
State the significance of binding energy per nucleon.
What is binding energy of nucleus?
