Advertisements
Advertisements
Question
Four solutions, namely glucose, alcohol, hydrochloric acid and sulphuric acid filled in four separate beakers are connected one by one in an electric circuit with a bulb. The solutions in which the bulb will glow when current is passed are ______.
Options
Glucose and alcohol
Alcohol and hydrochloric acid
Glucose and sulphuric acid
Hydrochloric acid and sulphuric acid
Solution
Four solutions, namely glucose, alcohol, hydrochloric acid and sulphuric acid filled in four separate beakers are connected one by one in an electric circuit with a bulb. The solutions in which the bulb will glow when current is passed are Hydrochloric acid and sulphuric acid.
APPEARS IN
RELATED QUESTIONS
State the differences between acids and bases.
How will you protect yourself from the heat generated while diluting a concentrated acid?
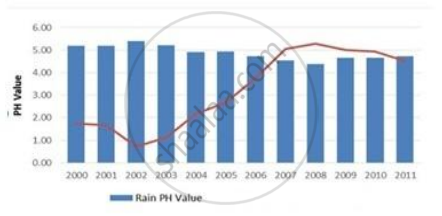
In which year is concentration of hydrogen ion the highest?
What will happen if we take dry HCl gas instead of the aqueous solution of HCl?
What will be the action of the following substances on blue litmus paper?
Dry HCl gas, Moistened NH3 gas, Lemon juice, Carbonated soft drink, Curd, Soap solution.
In one of the industrial processes used for the manufacture of sodium hydroxide, a gas X is formed as by product. The gas X reacts with lime water to give a compound Y which is used as a bleaching agent in chemical industry. Identify X and Y giving the chemical equation of the reactions involved.
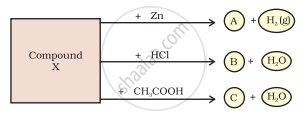
Identify the compound Y on the basis of the reactions given below. Also write the name and chemical formulae of A, B and C.
Suggest a safe procedure of diluting a strong concentrated acid.
Write the chemical equation for the following activity.
Dilute nitric acid was added to calcium oxide.
Write the chemical equation for the following activity.
Dilute nitric acid was added to calcium oxide.
