Advertisements
Advertisements
Question
In one of the industrial processes used for the manufacture of sodium hydroxide, a gas X is formed as by product. The gas X reacts with lime water to give a compound Y which is used as a bleaching agent in chemical industry. Identify X and Y giving the chemical equation of the reactions involved.
Solution
Sodium hydroxide is manufactured by the electrolysis of a strong solution of sodium chloride (called brine). As a result, chlorine (X) is evolved at anode while hydrogen at cathode. Chlorine reacts with lime water containing slaked lime to form bleaching powder (Y)
`underset(("Lime water"))("Ca"("OH")_2) + underset((X))("Cl"_2) -> underset("Bleaching Powder(Y)")("CaOCl"_2)+"H"_2"O"`
APPEARS IN
RELATED QUESTIONS
When hydrogen chloride gas is prepared on a humid day, the gas is usually passed through the guard tube containing calcium chloride. The role of calcium chloride taken in the guard tube is to:
What is formed when zinc reacts with sodium hydroxide?
Sodium hydroxide is a ____________.
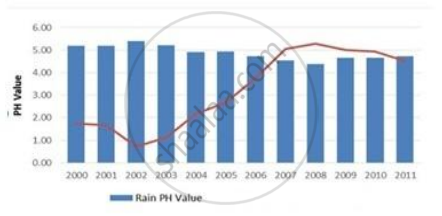
In which year is concentration of hydrogen ion the highest?
What will be the action of the following substances on blue litmus paper?
Dry HCl gas, Moistened NH3 gas, Lemon juice, Carbonated soft drink, Curd, Soap solution.
A dry pellet of a common base B, when kept in open absorbs moisture and turns sticky. The compound is also of chloralkali process. Identify B What type of reaction occurs when B is treated with an acidic oxide ? Write a balanced chemical equation for one such solution.
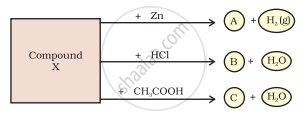
Identify the compound Y on the basis of the reactions given below. Also write the name and chemical formulae of A, B and C.
The change in colour of the moist litmus paper in the given set up is due to
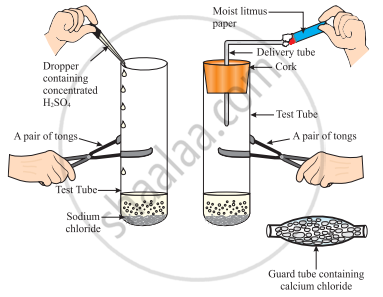
- presence of acid
- presence of base
- presence of H⁺(aq) in the solution
- presence of Litmus which acts as an indicator
Write the chemical equation for the following activity.
Dilute nitric acid was added to calcium oxide.
Write the chemical equation for the following activity.
Dilute nitric acid was added to calcium oxide.
