Advertisements
Online Mock Tests
Chapters
▶ 2: Acids, Bases and Salts
3: Metals and Non-metals
4: Carbon and its Compounds
5: Periodic Classification of Elements
6: Life Processes
7: Control and Coordination
8: How do Organisms Reproduce?
9: Heredity and Evolution
10: Light – Reflection and Refraction
11: The Human Eye and the Colourful World
12: Electricity
13: Magnetic Effects of Electric Current
14: Sources of Energy
15: Our Environment
16: Management of Natural Resources
![NCERT Exemplar solutions for Science [English] Class 10 chapter 2 - Acids, Bases and Salts NCERT Exemplar solutions for Science [English] Class 10 chapter 2 - Acids, Bases and Salts - Shaalaa.com](/images/science-english-class-10_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Advertisements
Solutions for Chapter 2: Acids, Bases and Salts
Below listed, you can find solutions for Chapter 2 of CBSE NCERT Exemplar for Science [English] Class 10.
NCERT Exemplar solutions for Science [English] Class 10 2 Acids, Bases and Salts Multiple Choice Questions [Pages 9 - 14]
What happens when a solution of an acid is mixed with a solution of a base in a test tube?
- The temperature of the solution increases
- The temperature of the solution decreases
- The temperature of the solution remains the same
- Salt formation takes place
(i) only
(i) and (iii)
(ii) and (iii)
(i) and (iv)
An aqueous solution turns red litmus solution blue. Excess addition of which of the following solutions would reverse the change?
Baking powder
Lime
Ammonium hydroxide solution
Hydrochloric acid
During the preparation of hydrogen chloride gas on a humid day, the gas is usually passed through the guard tube containing calcium chloride. The role of calcium chloride taken in the guard tube is to ______
absorb the evolved gas
moisten the gas
absorb moisture from the gas
absorb Cl– ions from the evolved gas
Which of the following salts does not contain any water of crystallisation?
Blue vitriol
Baking soda
Washing soda
Gypsum
Sodium carbonate is a basic salt because it is a salt of
strong acid and strong base
weak acid and weak base
strong acid and weak base
weak acid and strong base
Calcium phosphate is present in tooth enamel. Its nature is
basic
acidic
neutral
amphoteric
A sample of soil is mixed with water and allowed to settle. The clear supernatant solution turns the pH paper yellowish-orange. Which of the following would change the colour of this pH paper to greenish-blue?
Lemon juice
Vinegar
Common salt
An antacid
Which of the following gives the correct increasing order of acidic strength?
Water < Acetic acid < Hydrochloric acid
Water < Hydrochloric acid < Acetic acid
Acetic acid < Water < Hydrochloric acid
Hydrochloric acid < Water < Acetic acid
If a few drops of a concentrated acid accidentally spills over the hand of a student, what should be done?
Wash the hand with saline solution
Wash the hand immediately with plenty of water and apply a paste of sodium hydrogen carbonate
After washing hand with plenty of water, apply solution of sodium hydroxide on the hand
Neutralise the acid with a strong alkali
Sodium hydrogencarbonate when added to acetic acid evolves a gas. Which of the following statements are true about the gas evolved?
- It turns lime water milky
- It extinguishes a burning splinter
- It dissolves in a solution of sodium hydroxide
- It has a pungent odour
(i) and (ii)
(i), (ii) and (iii)
(ii), (iii) and (iv)
(i) and (iv)
Common salt besides being used in kitchen can also be used as the raw material for making
- washing soda
- bleaching powder
- baking soda
- slaked lime
(i) and (ii)
(i), (ii) and (iv)
(i) and (iii)
(i), (iii) and (iv)
One of the constituents of baking powder is sodium hydrogencarbonate. The other constituent is :
hydrochloric acid
tartaric acid
acetic acid
sulphuric acid
To protect tooth decay, we are advised to brush our teeth regularly. The nature of the tooth paste commonly used is
acidic
neutral
basic
corrosive
Which of the following statements is correct about an aqueous solution of an acid and of a base?
- Higher the pH, stronger the acid
- Higher the pH, weaker the acid
- Lower the pH, stronger the base
- Lower the pH, weaker the base
(i) and (iii)
(ii) and (iii)
(i) and (iv)
(ii) and (iv)
The pH of the gastric juices released during digestion is
less than 7
more than 7
equal to 7
equal to 0
Which of the following phenomena occur when a small amount of acid is added to water?
- Ionisation
- Neutralisation
- Dilution
- Salt formation
(i) and (ii)
(i) and (iii)
(ii) and (iii)
(ii) and (iv)
Which one of the following can be used as an acid- base indicator by a visually impared student?
Litmus
Turmeric
Vanilla essence
Petunia leaves
Which of the following substance will not give carbon dioxide on treatment with dilute acid?
Marble
Limestone
Baking soda
Lime
Which of the following is acidic in nature?
Lime juice
Human blood
Lime water
Antacid
In an attempt to demonstrate electrical conductivity through an electrolyte, the apparatus setup. Which among the following statement(s) is(are) correct?
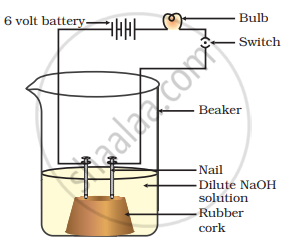
- Bulb will not glow because electrolyte is not acidic
- Bulb will glow because NaOH is a strong base and furnishes ions for conduction.
- Bulb will not glow because circuit is incomplete
- Bulb will not glow because it depends upon the type of electrolytic solution
(i) and (iii)
(ii) and (iv)
(ii) only
(iv) only
Which of the following is used for dissolution of gold?
Hydrochloric acid
Sulphuric acid
Nitric acid
Aqua regia
Which of the following is not a mineral acid?
Hydrochloric acid
Citric acid
Sulphuric acid
Nitric acid
Which of the following is not a base?
NaOH
KOH
NH4OH
C2H5OH
Which of the following statements is not correct?
All metal carbonates react with acid to give a salt, water and carbon dioxide
All metal oxides react with water to give salt and acid
Some metals react with acids to give salt and hydrogen
Some non metal oxides react with water to form an acid
Match the chemical substances given in Column (A) with their appropriate application given in Column (B)
| Column (A) |
Column (B) |
| (A) Bleaching powder | (i) Preparation of glass |
| (B) Baking soda | (ii) Production of H2 and Cl2 |
| (C) Washing Soda | (iii) Decolourisation |
| (D) Sodium chloride | (iv) Antacid |
A–(ii), B–(i), C–(iv), D–(iii) A–(iii), B–(ii), C–(iv), D–(i) A–(iii), B–(iv), C–(i), D–(ii) A–(ii), B–(iv), C–(i), D–(iii)
Equal volumes of hydrochloric acid and sodium hydroxide solutions of same concentration are mixed and the pH of the resulting solution is checked with a pH paper. What would be the colour obtained?

Red
Yellow
Yellowish Green
Blue
Which of the following is(are) true when HCl (g) is passed through water?
- It does not ionise in the solution as it is a covalent compound.
- It ionises in the solution
- It gives both hydrogen and hydroxyl ions in the solution
- It forms hydronium ion in the solution due to the combination of hydrogen ion with water molecule
(i) only
(iii) only
(ii) and (iv)
(iii) and (iv)
Which of the following statements is true for acids?
Bitter and change red litmus to blue
Sour and change red litmus to blue
Sour and change blue litmus to red
Bitter and change blue litmus to red
Which of the following are present in a dilute aqueous solution of hydrochloric acid?
H3O+ + Cl–
H3O+ + OH–
Cl– + OH–
unionised HCl
Identify the correct representation of reaction occurring during chloralkali process
`2"NaCl"("l") + 2"H"_2"O"("l") -> 2"NaOH"("l") + "Cl"_2("g") + "H"_2("g")`
`2"NaCl"("aq") + 2"H"_2"O"("aq") -> 2"NaOH"("aq") + "Cl"_2("g") + "H"_2("g")`
`2"NaCl"("aq") + 2"H"_2"O"("l") -> 2"NaOH"("aq") + "Cl"_2("aq") + "H"
_2("aq")``2"NaCl"("aq") + 2"H"_2"O"("l") -> 2"NaOH"("aq") + "Cl"_2("g") + "H"_2("g")`
NCERT Exemplar solutions for Science [English] Class 10 2 Acids, Bases and Salts Short Answer Questions [Pages 15 - 16]
Match the acids given in column (A) with their correct source given in column (B)
| Column (A) | Column (B) |
| (a) Lactic acid | Tamarind |
| (b) Acetic acid | Lemon |
| (c) Citric acid | Vinegar |
| (d) Tartaric acid | Curd |
Match the important chemicals given in Column (A) with the chemical formulae given in Column (B)
| Column (A) | Column (B) |
| (a) Plaster of Paris | Ca(OH)2 |
| (b) Gypsum | CaSO4. 1/2 H2O |
| (c) Bleaching Powder | CaSO4.2H7O |
| (d) Slaked Lime | CaOCl2 |
What will be the action of the following substances on blue litmus paper?
Dry HCl gas, Moistened NH3 gas, Lemon juice, Carbonated soft drink, Curd, Soap solution.
Name the acid present in ant sting and give its chemical formula. Also give the common method to get relief from the discomfort caused by the ant sting.
What happens when nitric acid is added to egg shell?
A student prepares solutions of (i) an acid and (ii) a base in two separate beakers. She forgot to label the solutions and litmus paper is not available in the laboratory. Since both the solutions are colourless, how will she distinguish between the two?
How would you distinguish between baking powder and washing soda by heating?
Salt A commonly used in bakery products on heating gets converted into another salt B which itself is used for the removal of hardness of water and a gas C is evolved. The gas C when passed through lime water, turns it milky. Identify A, B and C.
In one of the industrial processes used for the manufacture of sodium hydroxide, a gas X is formed as by product. The gas X reacts with lime water to give a compound Y which is used as a bleaching agent in chemical industry. Identify X and Y giving the chemical equation of the reactions involved.
Fill in the missing data in the following table
| Name of the salt | Formula | Salt obtained from | ||
| Base | Acid | |||
| (i) | Ammonium chloride | NH4Cl | NH4OH | ______ |
| (ii) | Copper sulphate | ______ | ______ | H2SO4 |
| (iii) | Sodium chloride | NaCl | NaOH | ______ |
| (iv) | Magnesium nitrate | Mg(NO3)2 | ______ | HNO3 |
| (v) | Potassium sulphate | K2SO4 | ______ | ______ |
| (vi) |
Calcium nitrate | Ca(NO3)2 | Ca(OH)2 | ______ |
What are strong and weak acids? In the following list of acids, separate strong acids from weak acids.
Hydrochloric acid, citric acid, acetic acid, nitric acid, formic acid, sulphuric acid.
When zinc metal is treated with a dilute solution of a strong acid, a gas is evolved which is utilised in the hydrogenation of oils. Name the gas evolved. Write the chemical equation of the reaction involved and also write a test to detect the gas formed.
NCERT Exemplar solutions for Science [English] Class 10 2 Acids, Bases and Salts Long Answer Questions [Pages 16 - 17]
In the following schematic diagram for the preparation of hydrogen gas as shown in the figure, what would happen if following changes are made?

- In place of zinc granules, same amount of zinc dust is taken in the test tube
- Instead of dilute sulphuric acid, dilute hydrochloric acid is taken
- In place of zinc, copper turnings are taken
- Sodium hydroxide is taken in place of dilute sulphuric acid and the tube is heated.
For making cake, baking powder is taken. If at home your mother uses baking soda instead of baking powder in cake,
- how will it affect the taste of the cake and why?
- how can baking soda be converted into baking powder?
- what is the role of tartaric acid added to baking soda?
A metal carbonate X on reacting with an acid gives a gas which when passed through a solution Y gives the carbonate back. On the other hand, a gas G that is obtained at anode during electrolysis of brine is passed on dry Y, It gives a compound Z, used for disinfecting drinking water. Identity X, Y, G and Z.
A dry pellet of a common base B, when kept in open absorbs moisture and turns sticky. The compound is also of chloralkali process. Identify B What type of reaction occurs when B is treated with an acidic oxide ? Write a balanced chemical equation for one such solution.
A sulphate salt of Group 2 element of the Periodic Table is a white, soft substance which can be moulded into different shapes by making its dough. When this compound is left in open for some time, it becomes a solid mass and cannot be used for moulding purposes. Identify the sulphate salt. Why does it show such a behaviour? Give the reaction involved.
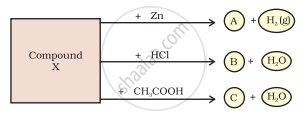
Identify the compound Y on the basis of the reactions given below. Also write the name and chemical formulae of A, B and C.
Solutions for 2: Acids, Bases and Salts
![NCERT Exemplar solutions for Science [English] Class 10 chapter 2 - Acids, Bases and Salts NCERT Exemplar solutions for Science [English] Class 10 chapter 2 - Acids, Bases and Salts - Shaalaa.com](/images/science-english-class-10_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
NCERT Exemplar solutions for Science [English] Class 10 chapter 2 - Acids, Bases and Salts
Shaalaa.com has the CBSE Mathematics Science [English] Class 10 CBSE solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. NCERT Exemplar solutions for Mathematics Science [English] Class 10 CBSE 2 (Acids, Bases and Salts) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. NCERT Exemplar textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Science [English] Class 10 chapter 2 Acids, Bases and Salts are Similarities and Differences Between Acids and Bases, Preparation and Uses of Sodium Hydroxide, Preparation and Uses of Bleaching Powder, Preparation and Uses of Baking Soda, Preparation and Uses of Washing Soda, Preparation and Uses of Plaster of Paris, Acids, Bases (Alkalis), Indicators, Properties of Acids, Properties of Bases (Alkalis), Acid or a Base in a Water Solution, Strength of Acidic or Basic Solutions, Salts, Important Salts in Daily Life.
Using NCERT Exemplar Science [English] Class 10 solutions Acids, Bases and Salts exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in NCERT Exemplar Solutions are essential questions that can be asked in the final exam. Maximum CBSE Science [English] Class 10 students prefer NCERT Exemplar Textbook Solutions to score more in exams.
Get the free view of Chapter 2, Acids, Bases and Salts Science [English] Class 10 additional questions for Mathematics Science [English] Class 10 CBSE, and you can use Shaalaa.com to keep it handy for your exam preparation.
