Advertisements
Advertisements
Question
What happens when a solution of an acid is mixed with a solution of a base in a test tube?
- The temperature of the solution increases
- The temperature of the solution decreases
- The temperature of the solution remains the same
- Salt formation takes place
Options
(i) only
(i) and (iii)
(ii) and (iii)
(i) and (iv)
Solution
(i) and (iv)
Explanation -
Salt formation takes place in the neutralisation reaction. It is always exothermic and temperature increases.
APPEARS IN
RELATED QUESTIONS
Metal compound A reacts with dilute hydrochloric acid to produce effervescence. The gas evolved extinguishes a burning candle. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride.
Why does an aqueous solution of an acid conduct electricity?
On adding dilute hydrochloric acid to copper oxide powder, the solution formed is blue-green.
On the basis of the above reaction, what can you say about the nature of copper oxide?
What are esters? How are esters prepared? Write the chemical equation for the reaction involved. What happens when an ester reacts with sodium hydroxide? Write the chemical equation for the reaction and also state the name and use of this reaction.
Choose the correct alternative and rewrite the following sentence.
Phenolphthalein is ___________ type of indicator.
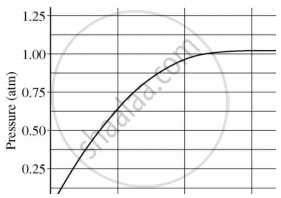
A student added 10 g of calcium carbonate in a rigid container, secured it tightly and started to heat it. After some time, an increase in pressure was observed, the pressure reading was then noted at intervals of 5 mins and plotted against time, in a graph as shown below. During which time interval did maximum decomposition take place?
Vinegar is ______ in taste.
Take a clean test tube with a holder and pour some dilute hydrochloric acid. Add a few pieces of magnesium ribbon pieces slowly. What do you observe? Now show a burning match stick near the mouth of the test tube. Do you hear any sound? The gas burns with a pop sound. From this, it is observed that hydrogen gas has been formed due to the reaction between acid and metal.
Consider the following salt:
NH4X
If in salt NH4X, X is nitrate, then its solution will give what colour with a universal indicator? Why?
The metal that will not produce hydrogen gas when reacted with dilute acids.
