Advertisements
Advertisements
Question
What are strong and weak acids? In the following list of acids, separate strong acids from weak acids.
Hydrochloric acid, citric acid, acetic acid, nitric acid, formic acid, sulphuric acid.
Solution
For the definitions of strong and weak acids and bases,
An acid may be defined as a substance which releases one or more H+ ions in aqueous solution. These ions exist as hydronium (H2O+) ions.
A base may be defined as a substance capable of releasing one or more OH– ions in aqueous solution.
In general, mineral acids are strong acids while organic acids are weak. From the available list :
Strong acids : Hydrochloric acid, nitric acid, sulphuric acid.
Weak acids : Citric acid, acetic acid, formic acid.
APPEARS IN
RELATED QUESTIONS
The litmus paper or the litmus solution is obtained from ________ plant
(A) Moss
(B) Lichen
(C) Rose
(D) Hibiscus
A first-aid manual suggests that vinegar should be used to treat wasp stings and baking soda for bee stings. What does this information tell you about the chemical nature of:
wasp stings?
Read the following statements:
I. When a red litmus paper is dipped into reaction mixture of a saponification reaction, it turns blue and the reaction is exothermic.
II. When a blue litmus paper is dipped into reaction mixture of a saponification reaction, its colour does not change and the reaction is exothermic.
III. When a red litmus paper is dipped into reaction mixture of a saponification reaction, its colour does not change and the reaction is endothermic.
IV. When a blue litmus paper is dipped into reaction mixture of a saponification reaction, its colour does not change and the reaction is endothermic.
Which of the above statements are correct:
(A) I, and II
(B) II and III
(C) III and IV
(D) I and IV
In an attempt to demonstrate electrical conductivity through an electrolyte, the apparatus setup. Which among the following statement(s) is(are) correct?
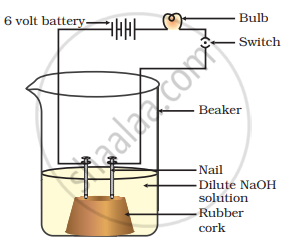
- Bulb will not glow because electrolyte is not acidic
- Bulb will glow because NaOH is a strong base and furnishes ions for conduction.
- Bulb will not glow because circuit is incomplete
- Bulb will not glow because it depends upon the type of electrolytic solution
Which of the following are present in a dilute aqueous solution of hydrochloric acid?
Solutions of acids conduct ______.
______ change the colour of the indicators.
Which acid is present in milk?
A substance 'X' is used as a building material and is insoluble in water. When it reacts with dil. HCl, it produces a gas which turns lime water milky.
- Write the chemical name and formula of 'X'.
- Write chemical equations for the chemical reactions involved in the above statements.
Some metals react with acids to produce salt and hydrogen gas. Illustrate it with an example. How will you test the presence of this gas?
