Advertisements
Advertisements
Question
Give a reason why –
Ozone is considered triatomic, but phosphorus a tetratomic molecule.
Solution
Ozone is considered triatomic because it is composed of three atoms while phosphorous is composed of four elements therefore it considered as tetratomic molecule.
APPEARS IN
RELATED QUESTIONS
Write down the name of a compound represented by the following formula:
Al2(SO4)3
Give the name of the element present in the following compound:
Baking powder
Give the name of the element present in the following compound:
Potassium sulphate
Define: Mass number :
Define: Atomic weight :
In what form do noble gases occur in nature?
What are (i) ionic compounds, and (ii) molecular compounds ? Give two examples of each type of compounds.
The cation of an element has :
Molecular compounds are usually formed by the combination between :
An element A forms an oxide A2O5.
What is the valency of element A ?
Calculate the mass of 3.011 × 1024 atoms of carbon.
Why are the symbols and formulae of substances important?
Explain the term – molecules. Give three examples of atoms of the same element forming a molecule. State the atomicity of the same.
Give one example of a triatomic molecule
State the main points of the contradiction of Dalton’s atomic theory by the Modern Atomic Theory.
State the atomicity of the following:
Oxygen
State the atomicity of the following:
Argon
State the atomicity of the following:
Sulphur
State true of false. If false, give the correct statement.
NaCl represents one molecule of sodium chloride.
Classify the following molecules as the molecules of element or compound
| 1. |  |
|
| 2. |  |
|
| 3. | 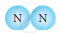 |
|
| 4. | 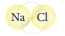 |
Which of the following is a triatomic molecule?
If a molecule is made of similar kinds of atoms, then it is called ______ atomic molecule.
Two elements sometimes can form more than one compound.
Define: Atomicity.
Derive the relationship between Relative molecular mass and Vapour density.
Write any two classifications of a molecule.
