Advertisements
Advertisements
Question
Give the disproportionation reaction of H3PO3
Solution
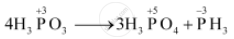
On heating, orthophosphorus acid (H3PO3) disproportionates to give orthophosphoric acid (H3PO4) and phosphine (PH3). The oxidation states of P in various species involved in the reaction are mentioned below.
APPEARS IN
RELATED QUESTIONS
Draw the structures of the following: H4P2O7 (Pyrophosphoric acid)
What is the basicity of H3PO4?
Complete the following equations : P4 + H2O →
Give reasons for the following : H3PO2 is a stronger reducing agent than H3PO3.
What are the oxidation states of phosphorus in H3PO3
What are the oxidation states of phosphorus in the following:
PCl3
Write balanced chemical equations involved in the following reactions:
Calcium phosphide is dissolved in water.
Phosphorus forms a number of oxoacids. Out of these oxoacids phosphinic acid has strong reducing property. Write its structure and also write a reaction showing its reducing behaviour.
Ortho phosphoric acid on heating gives:-
The oxidation number of phosphorus in Ba (H2PO3)2 is
Which one of the following is a dibasic acid?
Match List-I with List-II:
| List-I | List-II |
| name of oxo acid | Oxidation state of 'P' |
| (a) Hypophosphorous acid | (i) +5 |
| (b) Orthophosphoric acid | (ii) +4 |
| (c) Hypophosphoric acid | (iii) +3 |
| (d) Orthophosphorous acid | (iv) +2 |
| (v) +1 |
Choose the correct answer from the options given below:
What is the basicity of \[\ce{H3PO4}\]?
What is the basicity of H3PO4?
What is the basicity of \[\ce{H3PO4}\]?
What is the basicity of \[\ce{H3PO4}\]?
What is the basicity of H3PO4?
