Advertisements
Advertisements
Question
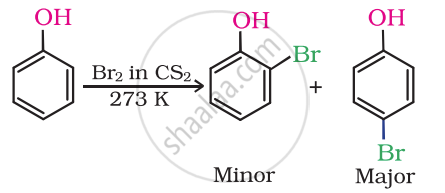
Give the equation of the following reaction:
Bromine in CS2 with phenol.
Solution
APPEARS IN
RELATED QUESTIONS
Write the final product(s) in each of the following reactions:
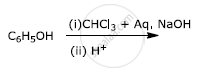
Write the main product(s) in each of the following reactions:
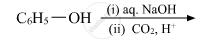
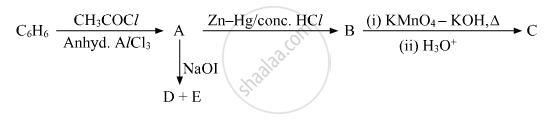
Write the structures of A, B, C, D and E in the following reactions:
Write the equation involved in the following reaction:
Kolbe’s reaction
Name the reagent used in the following reaction:
Bromination of phenol to 2, 4, 6-tribromophenol.
Write the reaction involved in the following:
Friedal-Crafts Alkylation of Phenol
Picric acid is ____________.
When phenol is treated with excess bromine water, it gives:
In the reaction of phenol with CHCl3 and aqueous NaOH at 343 K, the electrophile attacking the ring is:

Phenol does not undergo nucleophilic substitution reaction easily due to ______.
Out of o-nitrophenol and p-nitrophenol, which is more volatile? Explain.
Nitration is an example of aromatic electrophilic substitution and its rate depends upon the group already present in the benzene ring. Out of benzene and phenol, which one is more easily nitrated and why?
Which of the following reacts with phenol to give salicylaldehyde after hydrolysis?
Write the equations for the following reaction:
Phenol is treated with chloroform in the presence of NaOH
Why ortho-nitrophenol is steam volatile while para-nitrophenol is not?
For the pair phenol and cyclohexanol, answer the following:
Give one chemical test to distinguish between the two.
How can phenol be prepared from anisole? Give reaction.
