Advertisements
Advertisements
Question
How are 2 - nitropropane prepared from suitable oxime?
Solution
1-nitropropane and 2-nitropropane are prepared by oxidising propionaldehyde oxime and propan-2-one oxime with the help of trifluoroacetic acid.

APPEARS IN
RELATED QUESTIONS
How is 1-nitropropane prepared from suitable oxime?
Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. Give two reasons.
Why pKa of F-CH2-COOH is lower than that of Cl−CH2−COOH?
Distinguish between the following : Benzoic acid and methyl benzoate
CH3CO2H or CH2FCO2H
Which acid of the pair shown here would you expect to be stronger?
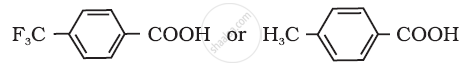
Arrange the following compounds in increasing order of their property as indicated:
CH3CH2CH(Br)COOH, CH3CH(Br)CH2COOH, (CH3)2CHCOOH, CH3CH2CH2COOH (acid strength)
Arrange the following compounds in increasing order of their property as indicated:
Benzoic acid, 4-Nitrobenzoic acid, 3, 4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)
Write the reactions involved Hell-Volhard Zelinsky reaction?
Account for the following:
Carboxylic acid is a stronger acid than phenol.
What happens when Salicylic acid is treated with (CH3CO)2 O/H+?
Arrange the following compounds in increasing order of their property as indicated:
F - CH2COOH, O2N - CH2 COOH CH3 COOH,HCOOH - acid character.
Complete the following reaction sequence.
\[\begin{array}{cc}
\ce{O}\phantom{...............................................}\\
||\phantom{...............................................}\\
\ce{CH3 - C - CH3 ->[(i) CH3MgBr][H2O] (A) ->[Na metal][Ether] (B) ->[CH3 - Br] (C)}
\end{array}\]
Assertion: Formaldehyde is a planar molecule.
Reason: It contains sp2 hybridised carbon atom.
Acidity of BF3 can be explained on the basis of which of the following concepts?
A mixture of benzaldehyde and formaldehyde on heating with 50% NaOH solution gives
When propionamide reacts with Br2 in the presence of alkali the product is ______.
Formic acid and formaldehyde can be distinguished by treating with ______.
Describe the action of alcoholic potassium hydroxide (alc. KOH) on ethyl bromide
Describe the action of alcoholic potassium hydroxide (alc. KOH) on n-propyl bromide
