Advertisements
Advertisements
Question
How is ethanol to ethanoic acid an oxidation reaction different from the reaction in which ethanol burns in the presence of oxygen?
Solution
The conversion of ethanol to ethanoic acid is considered an oxidation reaction because it involves adding oxygen to ethanol or removing hydrogen from ethanol, resulting in the formation of ethanoic acid.
This process increases the oxygen content or decreases the hydrogen content in the molecule, which is a characteristic of oxidation reactions.
APPEARS IN
RELATED QUESTIONS
What type of compound is formed when a carboxylic acid reacts with an alcohol in the presence of conc. H2SO4?
Choose those compounds from the following which can turn blue litmus solution red:
HCHO, CH3COOH, CH3OH, C2H5OH, HCOOH, CH3CHO
Give reasons for your choice.
What is an oxidising agent?
The structural formula of an ester is :

Write the formula of the acid and the alcohol from which it is formed.
Acetic acid is a typical acid. Write one equation in case of its reactions with a carbonate.
Name the following:
A toxic alcohol
What is the main constituent of vinegar?
The reaction between acetic acid and sodium carbonate is shown in the following figure.
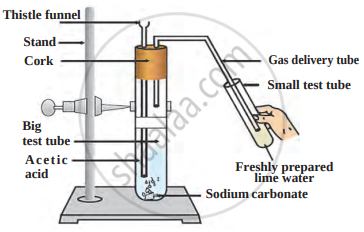
Answer the questions with the help of a diagram.
- Which gas does come out as effervescence in the big test tube?
- What is the colour change in the lime water present in the small test tube?
- Write the related reaction.
Raina while doing certain reactions observed that heating of substance ‘X’ with a vinegar-like smell with a substance ‘Y’ (which is used as an industrial solvent.) in the presence of conc. Sulphuric acid in a water bath gives a sweet-smelling liquid ‘Z’ having molecular formula C4H8O2. When heated with caustic soda (NaOH), ‘Z’ gives back the sodium salt and the compound ‘Y’.
Identify ‘X’, ‘Y’, and ‘Z’. Illustrate the changes with the help of suitable chemical equations.
Name the oxidising agent used in the conversion of ethanol to ethanoic acid.
