Advertisements
Advertisements
Question
Hydrogen gas is diatomic whereas inert gases are monoatomic – Explain on the basis of MO theory.
Solution
The molecular orbital electronic configuration of the hydrogen molecule is (σ1s2). The molecular orbital energy level diagram of the H2 molecule is given in
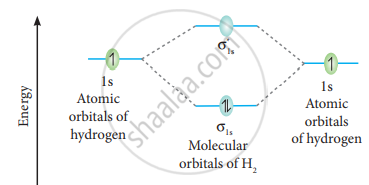
Here, N2 = 2, Na = 0
Bond order =
He2: σ1s2 σ1s*2
The molecular orbital energy level diagram of He2 (hypothetical) is given in
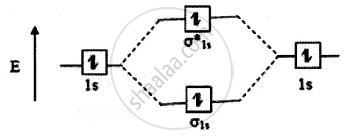
Here, Nb = 2 and Na = 2
Bond order =
As the bond order for He2 comes out to form between two hydrogen atoms. But as the bond order of helium is zero, there is no bond between helium atoms and hence it is monoatomic.
Result:
As the bond order of the H2 molecule is one, it is diatomic and a single bond is formed between two hydrogen atoms. But as the bond order of helium is zero, there is no bond between helium atoms and hence it is monoatomic.
APPEARS IN
RELATED QUESTIONS
Draw an orbital diagram of Hydrogen fluoride molecule
BF3 molecule is planar but NH3 pyramidal. Explain.
What are the interacting forces present during the formation of a molecule of a compound?
Assertion: Oxygen molecule is paramagnetic.
Reason: It has two unpaired electron in its bonding molecular orbital.
Of the following molecules, which have shape similar to carbon dioxide?
What do you understand by Linear combination of atomic orbitals in MO theory?
Which one of the following sets CORRECTLY represents the increase in the paramagnetic property of the ions?
According to MOT, the number of unpaired electrons in O2 molecule is ____________.
Of the ions Ti4+, Co2+ and Cr3+, ____________.
Bonding in which of the following diatomic molecule(s) become(s) stronger, on the basis of MO Theory, by removal of an electron?
(A) NO
(B) N2
(C) O2
(D) C2
(E) B2
Choose the most appropriate answer from the options given below:
