Advertisements
Advertisements
Question
In beta decay, an electron (or a positron) is emitted by a nucleus. Does the remaining atom get oppositely charged?
Solution
In beta decay, a neutron from the nucleus is converted to a proton releasing an electron and an antineutrino or a proton is converted to a neutron releasing a positron and a neutrino.
i.e. `β^(-) "decay" : n → p + e + bar(nu)`
`β^(+) "decay" : p → n + e^(+) + nu`
Since the number of valence electrons present in the parent atom do not change, the remaining atom does not get oppositely charged. Instead, due to a change in the atomic number, there's a formation of a new element.
APPEARS IN
RELATED QUESTIONS
For the `beta^+` (positron) emission from a nucleus, there is another competing process known as electron capture (electron from an inner orbit, say, the K−shell, is captured by the nucleus and a neutrino is emitted).
\[\ce{e+ + ^A_Z X -> ^A_{Z - 1}Y + \text{v}}\]
Show that if `beta^+` emission is energetically allowed, electron capture is necessarily allowed but not vice−versa.
Consider the D−T reaction (deuterium−tritium fusion)
\[\ce{^2_1H + ^3_1H -> ^4_2He + n}\]
Calculate the energy released in MeV in this reaction from the data:
`"m"(""_1^2"H")`= 2.014102 u
`"m"(""_1^3"H")`= 3.016049 u
Consider the D−T reaction (deuterium−tritium fusion)
\[\ce{^2_1H + ^3_1H -> ^4_2He}\]
Consider the radius of both deuterium and tritium to be approximately 2.0 fm. What is the kinetic energy needed to overcome the coulomb repulsion between the two nuclei? To what temperature must the gas be heated to initiate the reaction? (Hint: Kinetic energy required for one fusion event =average thermal kinetic energy available with the interacting particles = 2(3kT/2); k = Boltzman’s constant, T = absolute temperature.)
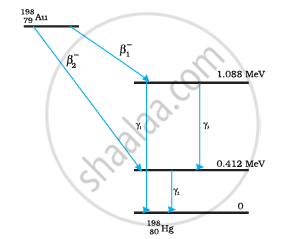
Obtain the maximum kinetic energy of β-particles, and the radiation frequencies of γdecays in the decay scheme shown in Fig. 13.6. You are given that
m (198Au) = 197.968233 u
m (198Hg) =197.966760 u
Write the basic nuclear process underlying β+ and β– decays.
Write the β-decay of tritium in symbolic form.
Write the basic nuclear process of neutron undergoing β-decay.
Why is the detection of neutrinos found very difficult?
What is the difference between cathode rays and beta rays? When the two are travelling in space, can you make out which is the cathode ray and which is the beta ray?
During a negative beta decay,
Consider a sample of a pure beta-active material.
A free neutron beta-decays to a proton with a half-life of 14 minutes. (a) What is the decay constant? (b) Find the energy liberated in the process.
(Use Mass of proton mp = 1.007276 u, Mass of `""_1^1"H"` atom = 1.007825 u, Mass of neutron mn = 1.008665 u, Mass of electron = 0.0005486 u ≈ 511 keV/c2,1 u = 931 MeV/c2.)
Complete the following decay schemes.
(a) `"" _88^226Ra → alpha+`
(b) `""_8^19O → _9^19F+`
(c) `""_13^25Al → ""_12^25Mg+`
A free neutron decays into ______.
