Advertisements
Advertisements
Question
In a cathode ray tube state:
(i) the purpose of covering cathode by thorium and carbon.
(ii) the purpose of the fluorescent screen.
(iii) how is it possible to increase the rate of emission of electrons.
Solution
(i) The cathode is covered with thorium and carbon as it has low work function and it needs to be heated up to 2000 K to emit electrons. This serves it as a better electron emitter than tungsten.
(ii) The fluorescent screen helps in changing the electrical signal applied on the deflecting plates into the visual pattern on the screen.
(iii) The rate of emission of the electrons can be increased by
- Using a metal with a lower work function,
- Increasing the temperature of the surface of the metal, or
- Increasing the surface area of the metal emitting the electrons.
APPEARS IN
RELATED QUESTIONS
What are free electrons?
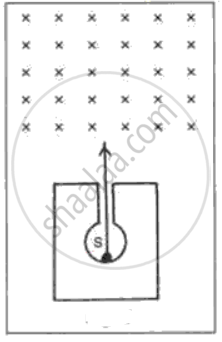
In following fig. shows a mixed source S of alpha and beta particles in a thick lead container. The particles pass through a magnetic field in a direction perpendicular to the plane of paper (inwards as shown by x). State and show in the diagram how the particles get affected.
Radioactive substances were found to give off three types of rays. Name them. How do they
(a) React to the magnetic field?
(b) React to the electric field?
(c) Act when different thickness of lead sheets is placed in their path?
During the emission of a beta particle, the ______ number remains same.
How is a cathode ray beam affected while passing through a magnetic field?
In a cathode ray tube state the purpose of covering cathode by thorium and carbon.
In a cathode ray tube state the purpose of the fluorescent screen.
What are ‘Becquerel rays’?
Name the Different Radiations Which Are Emitted by the Radioactive Substances.
Are all the radiations mentioned by you, emitted in a single radioactive decay?
