Advertisements
Advertisements
Question
In one average-life,
Options
half the active nuclei decay
less than half the active nuclei decay
more than half the active nuclei decay
all the nuclei decay.
Solution
more than half the active nuclei decay
The average life is the mean life time for a nuclei to decay.
It is given as `tau = 1/lambda = T_"1/2"/0.693`
Here, `tau , lambda and T_"1/2"`
are the average life, decay constant and half life-time of the active nuclei, respectively. The value of the average lifetime comes to be more than the average lifetime. Therefore, in one average life, more than half the active nuclei decay.
APPEARS IN
RELATED QUESTIONS
Asha's mother read an article in the newspaper about a disaster that took place at Chernobyl. She could not understand much from the articles and asked a few questions from Asha regarding the article. Asha tried to answer her mother's questions based on what she learnt in Class XII Physics.
(a) What was the installation at Chernobyl where the disaster took place? What according to you, was the cause of this disaster?
(b) Explain the process of release of energy in the installation at Chernobyl.
(c) What according to you, were the values displayed by Asha and her mother?
Draw the plot of binding energy per nucleon (BE/A) as a functino of mass number A. Write two important conclusions that can be drawn regarding the nature of nuclear force.
Using the curve for the binding energy per nucleon as a function of mass number A, state clearly how the release in energy in the processes of nuclear fission and nuclear fusion can be explained.
Suppose we have 12 protons and 12 neutrons. We can assemble them to form either a 24Mg nucleus or two 12C nuclei. In which of the two cases more energy will be liberated?
The mass number of a nucleus is equal to
As the mass number A increases, the binding energy per nucleon in a nucleus
Which of the following is a wrong description of binding energy of a nucleus?
Assume that the mass of a nucleus is approximately given by M = Amp where A is the mass number. Estimate the density of matter in kgm−3 inside a nucleus. What is the specific gravity of nuclear matter?
A neutron star has a density equal to that of the nuclear matter. Assuming the star to be spherical, find the radius of a neutron star whose mass is 4.0 × 1030 kg (twice the mass of the sun).
Calculate the mass of an α-particle. Its Its binding energy is 28.2 MeV.
(Use Mass of proton mp = 1.007276 u, Mass of `""_1^1"H"` atom = 1.007825 u, Mass of neutron mn = 1.008665 u, Mass of electron = 0.0005486 u ≈ 511 keV/c2,1 u = 931 MeV/c2.)
(a) Calculate the energy released if 238U emits an α-particle. (b) Calculate the energy to be supplied to 238U it two protons and two neutrons are to be emitted one by one. The atomic masses of 238U, 234Th and 4He are 238.0508 u, 234.04363 u and 4.00260 u respectively.
(Use Mass of proton mp = 1.007276 u, Mass of `""_1^1"H"` atom = 1.007825 u, Mass of neutron mn = 1.008665 u, Mass of electron = 0.0005486 u ≈ 511 keV/c2,1 u = 931 MeV/c2.)
What is the unit of mass when measured on the atomic scale?
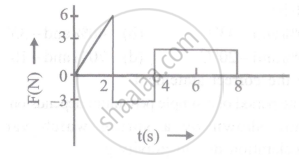
The force 'F' acting on a particle of mass 'm' is indicated by the force-time graph shown below. The change in momentum of the particle over the time interval from zero to 8s is:
A nucleus of mass M emits a γ-ray photon of frequency 'v'. The loss of internal energy by the nucleus is ______.
