Advertisements
Advertisements
Question
In which of the following decays the atomic number decreases?
(a) α-decay
(b) β+-decay
(c) β−-decay
(d) γ-decay
Solution
(a) α-decay
(b) β+-decay
In alpha particle decay, the unstable nucleus emits an alpha particle reducing its proton number (atomic number) Z as well as neutron number N by 2.
`""_Z^AX` → `""_(Z-2)^(A-4)Y` + `""_2^4He`
During β−-decay, a neutron is converted to a proton, an electron and an antineutrino. Thus, there is an increase in the atomic number.
`""_Z^AX` → `""_(Z+1)^(A)Y` + `e^-` +`barnu`
During β+-decay, a proton in the nucleus is converted to a neutron, a positron and a neutrino in order to maintain the stability of the nucleus. Thus, there is a decrease in the atomic number.
`""_Z^AX` → `""_(Z-1)^(A)Y` + `β^+` +`nu`
When a nucleus is in higher excited state or has excess of energy, it comes to the lower state in order to become stable and release energy in the form of electromagnetic radiation called gamma ray. The element in the gamma decay doesn't change.
Therefore, alpha and beta plus decay suffer decrease in atomic number.
APPEARS IN
RELATED QUESTIONS
Derive an expression for the total energy of electron in ‘n' th Bohr orbit. Hence show that energy of the electron is inversely proportional to the square of principal quantum number. Also define binding energy.
Write symbolically the nuclear β+ decay process of `""_6^11C` Is the decayed product X an isotope or isobar of (`""_6^11C`)? Given the mass values m (`""_6^11C`) = 11.011434 u and m (X) = 11.009305 u. Estimate the Q-value in this process.
Obtain the binding energy of the nuclei `""_26^56"Fe"` and `""_83^209"Bi"` in units of MeV from the following data:
`"m"(""_26^56"Fe")` = 55.934939 u
`"m"(""_83^209"Bi")`= 208.980388 u
What is the significance of binding energy per nucleon of a nucleus of a radioactive element?
Define the terms (i) half-life (T1/2) and (ii) average life (τ). Find out their relationships with the decay constant (λ).
How much energy is released in the following reaction : 7Li + p → α + α.
Atomic mass of 7Li = 7.0160 u and that of 4He = 4.0026 u.
(Use Mass of proton mp = 1.007276 u, Mass of `""_1^1"H"` atom = 1.007825 u, Mass of neutron mn = 1.008665 u, Mass of electron = 0.0005486 u ≈ 511 keV/c2,1 u = 931 MeV/c2.)
Find the binding energy per nucleon of `""_79^197"Au"` if its atomic mass is 196.96 u.
(Use Mass of proton mp = 1.007276 u, Mass of `""_1^1"H"` atom = 1.007825 u, Mass of neutron mn = 1.008665 u, Mass of electron = 0.0005486 u ≈ 511 keV/c2,1 u = 931 MeV/c2.)
Binding energy per nucleon for helium nucleus (2 He) is 7.0 MeV Find value of mass defect for helium nucleus
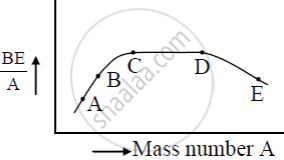
Sketch a graph showing the variation of binding energy per nucleon of a nucleus with its mass number.
The figure shows the plot of binding energy (BE) per nucleon as a function of mass number A. The letters A, B, C, D, and E represent the positions of typical nuclei on the curve. Point out, giving reasons, the two processes (in terms of A, B, C, D, and E ), one of which can occur due to nuclear fission and the other due to nuclear fusion.
An electron in hydrogen atom stays in its second orbit for 10−8 s. How many revolutions will it make around the nucleus at that time?
In a periodic table the average atomic mass of magnesium is given as 24.312 u. The average value is based on their relative natural abundance on earth. The three isotopes and their masses are\[\ce{_12^24Mg}\](23.98504 u), \[\ce{_12^25Mg}\] (24.98584 u), and \[\ce{_12^26Mg}\] (25.98259 u). The natural abundance of \[\ce{_12^24Mg}\] is 78.99% by mass. Calculate the abundances of other two isotopes.
A body's centre of mass
Tritium is an isotope of hydrogen whose nucleus Triton contains 2 neutrons and 1 proton. Free neutrons decay into `p + bare + barν`. If one of the neutrons in Triton decays, it would transform into He3 nucleus. This does not happen. This is because ______.
Find the binding energy of a H-atom in the state n = 2
State the significance of binding energy per nucleon.
Calculate the values of x and y in the following nuclear reaction.
\[\ce{^227_89Ac -> ^211_82Pb + x[^4_2He]+ y[^0_-1e]}\]
What is meant by “binding energy per nucleon” of a nucleus?
