Advertisements
Advertisements
Question
Match the type of unit cell given in Column I with the features given in Column II.
| Column I | Column II |
| (i) Primitive cubic unit cell | (a) Each of the three perpendicular edges compulsorily have the different edge length i.e; a ≠ b ≠ c. |
| (ii) Body centred cubic unit cell | (b) Number of atoms per unit cell is one. |
| (iii) Face centred cubic unit cell | (c) Each of the three perpendicular edges compulsorily have the same edge length i.e; a = b = c. |
| (iv) End centred orthorhombic cell | (d) In addition to the contribution from unit cell the corner atoms the number of atoms present in a unit cell is one. |
| (e) In addition to the contribution from the corner atoms the number of atoms present in a unit cell is three. |
Solution
| Column I | Column II |
| (i) Primitive cubic unit cell | (b) Number of atoms per unit cell is one. |
| (c) Each of the three perpendicular edges compulsorily have the same edge length i.e; a = b = c. | |
| (ii) Body-centred cubic unit cell | (c) Each of the three perpendicular edges compulsorily have the same edge length i.e; a = b = c. |
| (d) In addition to the contribution from unit cell the corner atoms the number of atoms present in a unit cell is one. | |
| (iii) Face centred cubic unit cell | (c) Each of the three perpendicular edges compulsorily have the same edge length i.e; a = b = c. |
| (e) In addition to the contribution from the corner atoms the number of atoms present in a unit cell is three. | |
| (iv) End-centred orthorhombic cell | (a) Each of the three perpendicular edges compulsorily have the different edge length i.e; a ≠ b ≠ c. |
| (d) In addition to the contribution from unit cell the corner atoms the number of atoms present in a unit cell is one. |
Explanation:
(i) 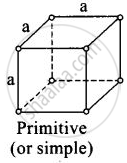
For primitive unit cell, a = b = c
Total number of atoms per unit cell = `1/8 xx 8` = 1
Here, `1/8` is due to contribution of each atom present at comer.
(ii)
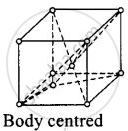
For body centered cubic unit cell, a = b = c.
This lattice contains atoms at comer as well as body centre.
Contribution due to atoms at comer = `1/8 xx 8` = 1
Contribution due to atoms at body centre = 8
(iii)

For face centred unit cell, a = b = c
Total constitutent ions per cell present at corners = `1/8 xx 8` = 1
Total constitutent ions per unit cell present at face centre = `1/2 xx 6` = 3
(iv)
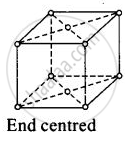
For end centred orthorhombic unit cell, a ≠ b ≠ c
Total contribution of atoms present at corner = `1/8 xx 8` = 1
Total constibution of atoms present at end centre = `1/2 xx 2` = 1
APPEARS IN
RELATED QUESTIONS
Face centred cubic crystal lattice of copper has density of 8.966 g.cm-3. Calculate the volume of the unit cell. Given molar mass of copper is 63.5 g mol-1 and Avogadro number NA is 6.022 x 1023 mol-1
An element crystallises in a b.c.c lattice with cell edge of 500 pm. The density of the element is 7.5g cm-3. How many atoms are present in 300 g of the element?
Distinguish between Hexagonal and monoclinic unit cells
Calculate the percentage efficiency of packing in case of simple cubic cell.
Number of types of orthorhombic unit cell is ___________.
An atom located at the body center of a cubic unit cell is shared by ____________.
An element forms a cubic unit cell with edge length 405 pm. Molar mass of this element is 2.7 × 10−2 kg/mol and its density is given as 2.7 × 103 kg/m3. How many atoms of these elements are present per unit cell?
Gold has a face-centered cubic lattice with an edge length of the unit cube of 407 pm. Assuming the closest packing, the diameter of the gold atom is ____________.
The density of a metal which crystallises in bcc lattice with unit cell edge length 300 pm and molar mass 50 g mol−1 will be:
The coordination number for body center cubic (BCC) system is
