Advertisements
Advertisements
Question
Name the following: A compound added to lower the fusion temperature of electrolytic bath in the extraction of aluminum
Solution
Cryolite (Na3AlF6)
APPEARS IN
RELATED QUESTIONS
Explain the following:
In construction work, why is the alloy of aluminium-duralumin used rather than pure aluminium?
Aluminium is extracted from its chief ore, bauxite. The ore is first purified and then the metal is extracted from it by electrolytic reduction.
Write an equation for the reaction which takes place at the anode during the extraction of aluminium by the electrolytic process.
How is ore purified (give equations also)
For the substance listed below, explain its role in the extraction of aluminium: Sodium hydroxide
For the substance listed below, explain its role in the extraction of aluminium: Graphite
Aluminium is extracted from its chief ore bauxite. The ore is first purified and then the metal is extracted from it by electrolytic reduction.
Write an equation for the reaction which takes place at the anode during the extraction of aluminium by the electrolytic process.
Describe the role played in the extraction of aluminum:
Cryolite
Name the alloy used for the following purpose.
Making medals
Answer the following question:
Write the chemical formula of one main ore of iron and aluminium.
The following sketch illustrates the process of conversion of Alumina to Aluminium:
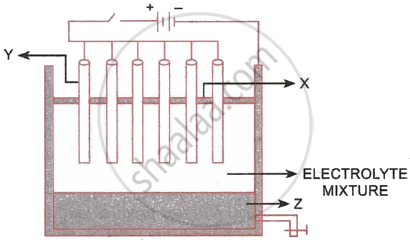
- Name the constituent of the electrolyte mixture which has a divalent metal in it.
- Name the powdered substances ‘X’ sprinkled on the surface of the electrolyte mixture.
- What is the name of the process?
- Write the reactions taking place at the electrodes ‘Y’ (anode) and ‘Z’ (cathode), respectively.
