Advertisements
Advertisements
Question
Name a non-metallic element which is a conductor of electricity.
Solution
Carbon in the form of graphite
APPEARS IN
RELATED QUESTIONS
Name the compound added to pure alumina to lower the fusion temperature during the electrolytic reduction of alumina.
Write a balanced chemical equation for each of the following reactions :
(i) Reduction of copper (II) oxide by hydrogen
Give equation for the following conversion: Aluminium hydroxide to aluminium oxide.
Give equation for the following conversion: Aluminium to aluminium nitride.
Give equation for the following conversion:Aluminium to sodium aluminate.
Name oxide of one metal which is reduced by (give equation): Reduction with heat alone
Write balanced equation for the following reaction:
Reduction of lead (II) oxide by carbon.
4 tones of bauxite, 150 Kg of sodium hydroxide and 600 Kg of graphite.
The aluminium compound in bauxite is aluminium oxide and the main impurity is iron (II) oxide. Aluminium is obtained by the electrolysis of aluminium oxide dissolved in cryolite.
(a) When bauxite is treated with sodium hydroxide solution, what happens to:
(i) the aluminium oxide?
(ii) The iron (III) oxide?
Name two metallic oxides which cannot be reduced by carbon, carbon monoxide or hydrogen.
The following sketch represents the electroplating of an Iron cup with Nickel metal.
Study the diagram and answer the following questions:
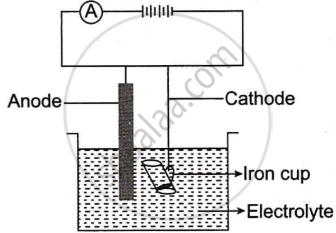
- During electroplating, the iron cup is placed at the cathode. Why?
- Name the ion that must be present in the electrolyte.
- State one condition that is necessary to ensure that the deposit is smooth, firm and even.
- Write the reaction taking place at the cathode.
- What change would you observe at the anode?
