Advertisements
Advertisements
Question
Name a non-metallic element which has a metallic lustre
Solution
Iodine
APPEARS IN
RELATED QUESTIONS
How is the following metallic oxide reduced? Write the equation:
Zinc oxide
Name oxide of one metal which is reduced by (give equation): Electrolytic reduction
For the reaction of a metal oxide. Suggest a reducing agent other than carbon
Give the chemical name and formula of 'cryolite'
Name a non-metallic element which forms acidic and neutral oxides
Name a non-metallic element which is a conductor of electricity.
What metallic property is shown by the non-metal graphite?
Complete the following by selecting the correct option from the choices given :
The metal whose oxide, which is amphoteric, is reduced to metal by carbon reduction ________
Complete the following by selecting the correct option from the choices given :
The diavalent metal whose oxide is reduced to metal by electrolysis of its fused salt is ________
The following sketch represents the electroplating of an Iron cup with Nickel metal.
Study the diagram and answer the following questions:
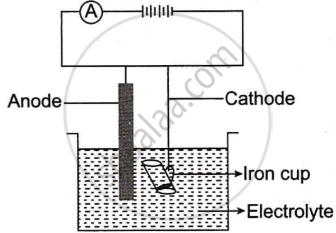
- During electroplating, the iron cup is placed at the cathode. Why?
- Name the ion that must be present in the electrolyte.
- State one condition that is necessary to ensure that the deposit is smooth, firm and even.
- Write the reaction taking place at the cathode.
- What change would you observe at the anode?
