Advertisements
Advertisements
Question
Name the three sub-atomic particles of an atom.
Solution
Name the three subatomic particles of an atom:
- Electron
- Proton
- Neutron
APPEARS IN
RELATED QUESTIONS
Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Compare the properties of electrons, protons and neutrons.
The mass of an electron is about `1/2000` times that of proton.
Fill in the blank of the following statement :
If the nucleus of an atom has atomic number 17, mass number 37 and there are 17 electrons outside the nucleus, the number of neutrons in it is __________.
What is a neutron? State its relative mass and charge.
Sulphur has an atomic number of 16 and a mass number of 32. State the number of protons and neutrons present in the nucleus of sulphur.
Write down the number of neutrons in the nucleus of an atom having atomic number 17 and mass number 37.
State the number of neutrons in each of the atoms A to E. Also state which of the atoms A to E is a metal.
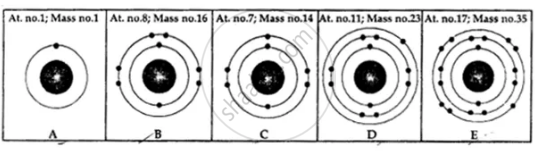
Calculate the number of neutrons, protons and electrons:
- atomic number 3 and mass number 7
- atomic number 92 and mass number 238.
The hydrogen atom does not have ______.
