Advertisements
Advertisements
Question
Give the formula and describe the structure of a noble gas species which is isostructural with
`ICI_4^(-)`
Solution 1
XeF4 is isoelectronic with `ICI_4^(-)` and has square planar geometry.
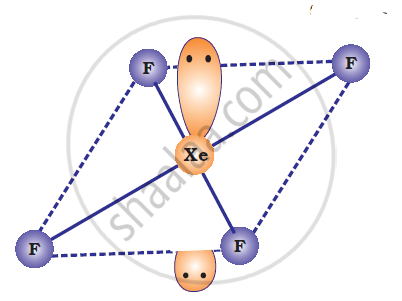
Solution 2
ICI4–: In ICI4–, central atom I has seven valence electrons and one due to negative charge. Four out of these 8 electrons are utilized in forming four single bonds with four Cl atoms. Four remaining electrons constitutes the two lone pairs. It is arranged in square planar structure. ICI4– has 36 valence electrons. A noble gas species having 36 valence electrons is XeF4 (8 + 4 x 7 = 36). XeF4 is also square planar.
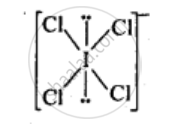
APPEARS IN
RELATED QUESTIONS
Account for the following:
Helium is used in diving apparatus.
What happens when: XeF4 reacts with SbF5?
Which noble gas is used in filling balloons for meteorological observations?
Write the structures of the following molecules: XeOF4
Draw the structures of the following:
XeO3
Why has it been difficult to study the chemistry of radon?
How are xenon fluorides XeF2, XeF4 and XeF6 obtained?
Which one of the following does not exist?
(i) XeOF4
(ii) NeF2
(iii) XeF2
(iv) XeF6
Complete the following reactions : XeF6 + 3H2O →
Write the electronic configuration of the following element:
Krypton (Z = 36)
Draw the structure of XeF4.
Sulfur dioxide reacts with sodium hydroxide solution to form _______.
Draw structure and name the shape of bromine trifluoride.
Which of the following fluorides does not exist?
Helium is preferred to be used in balloons instead of hydrogen because it is ____________.
In which of the following pairs, the two species are isostructural:
Which of the following reactions is an example of redox reaction?
The order of increasing sizes of atomic radii among the elements O, S, Se and As is:
Noble gases are named because of their inertness towards reactivity. Identify an incorrect statement about them.
\[\ce{XeF6 + H2O ->[Partial][Hydrolysis] \underline{}\underline{}\underline{}\underline{} + \underline{}\underline{}\underline{}\underline{}}\]
