Advertisements
Advertisements
Question
On the basis of VB theory explain the nature of bonding in \[\ce{[Co(C2O4)3]^3-}\].
Solution
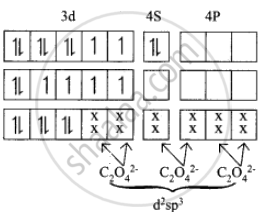
In the complex entity \[\ce{[Co(C2O4)3]^3-}\] the Co is in +3 oxidation state. The outer electronic configuration of Co3+ is 3d6. The oxalato ligand is fairly strong field ligand. So it faces the 3d electrons in Co3+ to pair up and make two of the 3d orbitals available for bonding. As a result, Co3+ shows d2sp2 hybridisation. Electronic configuration of Co atom Electronic configuration of Co3+ ion Hybridisation and formation of \[\ce{[Co(C2O4)3]^3-}\]
1. There is no unpaired electron in \[\ce{[Co(C2O4)3]^3-}\] Thus \[\ce{[Co(C2O4)3]^3-}\]
2. During the formtion of \[\ce{[Co(C2O4)3]^3-}\], two of the 3d-orbitals are used in bonding. Therefore it is an inner orbital (low spin) complex.
3. The \[\ce{[Co(C2O4)3]^3-}\] has the octahedral geometry.
APPEARS IN
RELATED QUESTIONS
Oxidation state of Iron and the charge on the ligand NO in [Fe(H2O)5NO]SO4 are ____________.
In which of the following coordination entities the magnitude of Δ0 will be maximum?
Give an example of coordination compound used in medicine and two examples of biologically important coordination compounds.
Based on VB theory explain why \[\ce{[Cr(NH3)6]^3+}\] is paramagnetic, while \[\ce{[Ni(CN)4]^2-}\] is diamagnetic.
[Ti(H2O)6]3+ is coloured, while [Sc(H2O)6]3+ is colourless – explain.
Classify the following ligand based on the number of donor atoms.
NH3
Classify the following ligand based on the number of donor atoms.
en
Classify the following ligand based on the number of donor atoms.
ox2−
Classify the following ligand based on the number of donor atoms.
pyridine
What is the coordination entity formed when excess of liquid ammonia is added to an aqueous solution of copper sulphate?
