Advertisements
Advertisements
Question
Show how will you synthesize cyclohexylmethanol using an alkyl halide by an SN2 reaction.
Solution 1
When chloromethylcyclohexane is treated with sodium hydroxide, cyclohexylmethanol is obtained.
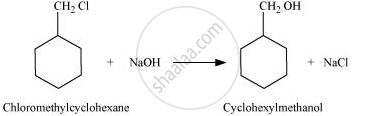
Solution 2
Hydrolysis of cyclohexylmethyl bromide by aqueous NaOH gives cyclohexylmethanol.
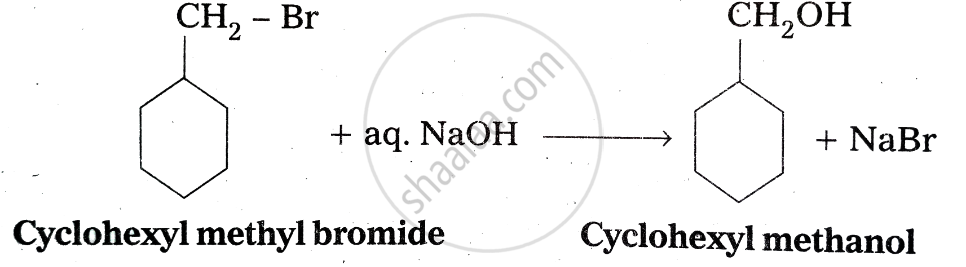
Notes
Students can refer to the provided solutions based on their preferred marks.
APPEARS IN
RELATED QUESTIONS
Write the main product(s) in each of the following reactions:
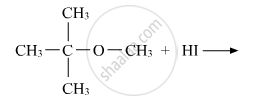
Predict the product of the following reaction:
\[\ce{CH3 - CH2 - CH2 - O - CH3 + HBr ->}\]
Predict the product of the following reaction:
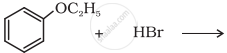
Write the equation of the reaction of hydrogen iodide with methoxybenzene.
Write the equation of the reaction of hydrogen iodide with benzyl ethyl ether.
Explain the fact that in aryl alkyl ethers
- the alkoxy group activates the benzene ring towards electrophilic substitution and
- it directs the incoming substituents to ortho and para positions in the benzene ring.
Write the product(s) in the following reaction
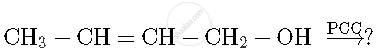
Write the formula of reagents used in the following reactions :
Bromination of phenol to 2,4,6-tribromophenol
Write the formula of reagents used in the following reactions :
Hydroboration of propene and then oxidation to propanol.
Write the structures of the main products in the following reactions :
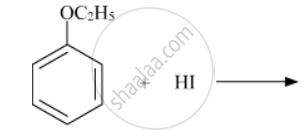
The ether
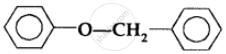
when treated with HI produces:
Maximum number of H-bonds that can be formed by a water molecule is
Indicate the σ and π bond in the following molecule:
HCONHCH3
